6. TREATMENT
This chapter reviews the available treatment modalities, followed by separate sections addressing treatment for the various disease stages.
6.1. Estimating life expectancy and health status
6.1.1. Introduction
Evaluation of life expectancy and health status is important in clinical decision-making for early detection, diagnosis, and treatment of PCa. Prostate cancer is common in older men (median age 68 years) and diagnoses in men > 65 years will result in a 70% increase in annual diagnosis by 2030 in Europe and the USA [493,494].
Active treatment mostly benefits patients with intermediate- or high-risk PCa and longest expected survival. In localised disease, over ten years life expectancy is considered mandatory for any benefit from local treatment and an improvement in CSS may take longer to become apparent. Older age and worse baseline health status have been associated with a smaller benefit in PCSM and life expectancy of surgery vs. AS [495]. Although in a RCT the benefit of surgery with respect to death from PCa was largest in men < 65 years of age (RR: 0.45), RP was associated with a reduced risk of metastases and use of androgen deprivation therapy (ADT) also among older men (RR: 0.68 and 0.60, respectively) [496]. External beam RT shows similar cancer control regardless of age, assuming a dose of > 72 Gy when using intensity-modulated or image-guided RT [497].
Older men have a higher incidence of PCa and may be under-treated despite the high overall mortality rates [498,499]. Of all PCa-related deaths 71% occur in men aged > 75 years [500], probably due to the higher incidence of advanced disease and death from PCa despite higher death rates from competing causes [501-503]. In the USA, only 41% of patients aged > 75 years with intermediate- and high-risk disease received curative treatment compared to 88% aged 65–74 [504].
6.1.2. Life expectancy
Life expectancy tables for European men are available online: https://ec.europa.eu/eurostat/. Survival may be variable and therefore estimates of survival must be individualised. Gait speed is a good single predictive method of life expectancy (from a standing start, at usual pace, generally over 6 meters). For men at age 75, ten-year survival ranged from 19% < 0.4 m/s to 87%, for ≥ 1.4 m/s [505].
Figure 6.1: Predicted Median Life Expectancy by Age and Gait Speed for males* [505]
 *Figure reproduced with permission of the publisher, from Studenski S, et al. JAMA 2011 305(1)50.
*Figure reproduced with permission of the publisher, from Studenski S, et al. JAMA 2011 305(1)50.
6.1.3. Health status screening
Heterogeneity in performance increases with advancing age, so it is important to use measures other than just age or performance status (PS) when considering treatment options. The International SIOG PCa Working Group recommends that treatment for adults over 70 years of age should be based on a systematic evaluation of health status using the G8 (Geriatric 8) screening tool (Table 6.1.1) [146]. This tool helps to discriminate between those who are fit and those with frailty, a syndrome of reduced ability to respond to stressors. Patients with frailty have a higher risk of mortality and negative side effects of cancer treatment [506]. Healthy patients with a G8 score > 14 or vulnerable patients with reversible impairment after resolution of their geriatric problems should receive the same treatment as younger patients. Frail patients with irreversible impairment should receive adapted treatment. Patients who are too ill should receive only palliative treatment (see Figure 5.3) [146]. Patients with a G8 score ≤ 14 should undergo a comprehensive geriatric assessment (CGA) as this score is associated with three-year mortality. A CGA is a multi-domain assessment that includes co-morbidity, nutritional status, cognitive and physical function, and social supports to determine if impairments are reversible [507]. A SR of the effect of geriatric evaluation for older cancer patients showed improved treatment tolerance and completion [508].
The Clinical Frailty Scale (CFS) is another screening tool for frailty (see Figure 5.4) [509]. Although not frequently used in the cancer setting, it is considered to be a common language for expressing degree of frailty. The scale runs from one to nine, with higher scores indicating increasing frailty. Patients with a higher CFS score have a higher 30-day mortality after surgery and are less likely to be discharged home [510].
It is important to use a validated tool to identify frailty, such as the G8 or CFS, as clinical judgement has been shown to be poorly predictive of frailty in older patients with cancer [511].
6.1.3.1. Co-morbidity
Co-morbidity is a major predictor of non-cancer-specific death in localised PCa treated with RP and is more important than age [512,513]. Ten years after watchful waiting for PCa, most men with a high co-morbidity score had died from competing causes, irrespective of age or tumour aggressiveness [512]. Measures for co-morbidity include: Cumulative Illness Score Rating-Geriatrics (CISR-G) [514,515] (Table 6.1.2) and Charlson Co-morbidity Index (CCI) [516].
6.1.3.2. Nutritional status
Malnutrition can be estimated from body weight during the previous three months (good nutritional status < 5% weight loss; risk of malnutrition: 5–10% weight loss; severe malnutrition: > 10% weight loss) [517].
6.1.3.3. Cognitive function
Cognitive impairment can be screened for using the mini-COG (https://mini-cog.com/) which consists of three-word recall and a clock-drawing test and can be completed within five minutes. A score of ≤ 3/5 indicates the need to refer the patient for full cognitive assessment. Patients with any form of cognitive impairment (e.g., Alzheimer’s or vascular dementia) may need a capacity assessment of their ability to make an informed decision, which is an increasingly important factor in health status assessment [518-520]. Cognitive impairment also predicts risk of delirium, which is important for patients undergoing surgery [521].
6.1.3.4. Physical function
Measures for overall physical functioning include: Karnofsky score and ECOG scores [522]. Measures for dependence in daily activities include Activities of Daily Living (ADL; basic activities) and Instrumental Activities of Daily Living (IADL; activities requiring higher cognition and judgement) [523-525].
6.1.3.5. Shared decision-making
The patient’s own values and preferences should be considered as well as the above factors. A shared decision-making process also involves anticipated changes to QoL, functional ability, and a patient’s hopes, worries and expectations about the future [526]. Particularly in older and frail patients, these aspects should be given equal importance to disease characteristics during the decision-making process [527]. Older patients may also wish to involve family members, and this is particularly important where cognitive impairment exists.
6.1.4. Conclusion
Individual life expectancy, health status, frailty, and co-morbidity, not only age, should be central in clinical decisions on screening, diagnostics, and treatment for PCa. A life expectancy of ten years is most commonly used as a threshold for benefit of local treatment. Older men may be undertreated. Patients aged 70 years of age or older who have frailty should receive a comprehensive geriatric assessment. Resolution of impairments in vulnerable men allows a similar urological approach as in fit patients.
Table 6.1.1: G8 s Predictcreening tool (adapted from ) [528]
| Items | Possible responses (score) | |
| A | Has food intake declined over the past three months due to loss of appetite, digestive problems, chewing, or swallowing difficulties? | 0 = severe decrease in food intake |
| 1 = moderate decrease in food intake | ||
| 2 = no decrease in food intake | ||
| B | Weight loss during the last three months? | 0 = weight loss > 3 kg |
| 1 = does not know | ||
| 2 = weight loss between 1 and 3 kg | ||
| 3 = no weight loss | ||
| C | Mobility? | 0 = bed or chair bound |
| 1 = able to get out of bed/chair but does not go out | ||
| 2 = goes out | ||
| D | Neuropsychological problems? | 0 = severe dementia or depression |
| 1 = mild dementia | ||
| 2 = no psychological problems | ||
| E | BMI? (weight in kg)/(height in m2) | 0 = BMI < 19 |
| 1 = BMI 19 to < 21 | ||
| 2 = BMI 21 to < 23 | ||
| 3 = BMI > 23 | ||
| F | Takes more than three prescription drugs per day? | 0 = yes |
| 1 = no | ||
| G | In comparison with other people of the same age, how does the patient consider his/her health status? | 0.0 = not as good |
| 0.5 = does not know | ||
| 1.0 = as good | ||
| 2.0 = better | ||
| H | Age | 0 = > 85 |
| 1 = 80-85 | ||
| 2 = < 80 | ||
| Total score | 0-17 |
Figure 6.2: Decision tree for health status screening (men > 70 years)** [146]

Mini-COGTM = Mini-COGTM cognitive test; ADLs = activities of daily living; CIRS-G = Cumulative IllnessRating Score - Geriatrics; CGA = comprehensive geriatric assessment.
* For Mini-COGTM, a cut-off points of ≤ 3/5 indicates a need to refer the patient for full evaluation of potential dementia.
**Reproduced with permission of Elsevier, from Boyle H. J., et al. Eur J Cancer 2019:116; 116 [146]
Figure 6.3: The Clinical Frailty Scale version 2.0 [509]*
 *Permission to reproduce the CFS was granted by the copyright holder.
*Permission to reproduce the CFS was granted by the copyright holder.
Table 6.2: Cumulative Illness Score Rating-Geriatrics (CISR-G)
| 1 | Cardiac (heart only) |
| 2 | Hypertension (rating is based on severity; affected systems are rated separately) |
| 3 | Vascular (blood, blood vessels and cells, marrow, spleen, lymphatics) |
| 4 | Respiratory (lungs, bronchi, trachea below the larynx) |
| 5 | ENT (eye, ear, nose, throat, larynx) |
| 6 | Upper GI (oesophagus, stomach, duodenum. Biliar and pancreatic trees; do not include diabetes) |
| 7 | Lower GI (intestines, hernias) |
| 8 | Hepatic (liver only) |
| 9 | Renal (kidneys only) |
| 10 | Other GU (ureters, bladder, urethra, prostate, genitals) |
| 11 | Musculo-Skeletal-Integumentary (muscles, bone, skin) |
| 12 | Neurological (brain, spinal cord, nerves; do not include dementia) |
| 13 | Endocrine-Metabolic (includes diabetes, diffuse infections, infections, toxicity) |
| 14 | Psychiatric/Behavioural (includes dementia, depression, anxiety, agitation, psychosis) |
All body systems are scores on a 0 - 4 scale. - 0: No problem affecting that system. - 1: Current mild problem or past significant problem. - 2: Moderate disability or morbidity and/or requires first line therapy. - 3: Severe problem and/or constant and significant disability and/or hard to control chronic problems. - 4: Extremely severe problem and/or immediate treatment required and/or organ failure and/or severe functional impairment. | |
| Total score 0-56 | |
6.1.5. Guidelines for evaluating health status and life expectancy
| Recommendations | Strength rating |
| Use individual life expectancy, health status, and co-morbidity in PCa management. | Strong |
| Use the Geriatric-8, mini-COG and Clinical Frailty Scale tools for health status screening. | Strong |
| Perform a full specialist geriatric evaluation in patients with a G8 score ≤ 14. | Strong |
| Consider standard treatment in vulnerable patients with reversible impairments (after resolution of geriatric problems) similar to fit patients, if life expectancy is > 10 years. | Weak |
| Offer adapted treatment or watchful waiting to patients with irreversible impairment. | Weak |
| Offer palliative symptom-directed therapy alone to frail patients. | Strong |
6.2. Treatment modalities
6.2.1. Expectant management strategies
Two different strategies of expectant management exist. For PCa in which curative therapy (using surgery or radiation) is not possible or indicated and palliative hormonal therapy not yet indicated, may be followed until local or metastatic symptomatic progression, to delay the side effects of androgen deprivation therapy (ADT). This strategy is referred to as watchful waiting (WW).
In patients with low- to intermediate-risk PCa, curative therapy may be postponed, or avoided altogether, using AS. As the prevalence of cancer cells in the prostate is so much higher than the risk of dying from PCa, together with the increased rate of early detection of small tumours after the introduction of PSA, there is a distinct risk of over-diagnosis and subsequent over-treatment of the disease (Chapter 3.1 Epidemiology) [9,529,530]. At the same time all available radical PCa treatment options may cause significant side effects. The differences between WW and AS are presented in Table 6.2.1.
Table 6.2.1: Differences between active surveillance and watchful waiting [478]
| Active surveillance | Watchful waiting | |
| Treatment intent | Curative | Palliative |
| Follow-up | Pre-defined schedule | Patient-specific |
| Assessment/markers* used | DRE, PSA, re-biopsy, imaging (MRI) | • None (wait for symptoms); or • Annual/biannual PSA (consider DRE if significant PSA-rise or imaging if metastases suspected) |
| Life expectancy | > 10 years | < 10 years |
| Aim | Minimise curative treatment-related toxicity without compromising survival, as the PCa is so indolent that it is unlikely to cause symptoms even with long life expectancy | Minimise palliative treatment-related (ADT) toxicity without compromising survival, PCa is unlikely to affect lifespan. |
| Eligible patients | Low- and selected intermediate-risk patients | Can apply to patients in all risk groups |
DRE = digital rectal examination; PSA = prostate-specific antigen; MRI = magnetic resonance imaging.
*Molecular markers and/or PSMA-PET/CT (-MRI) may be used.
Data from studies conducted on patients who did not undergo local treatment with up to 25 years of follow-up, with endpoints of OS and CSS, are available. Several series have shown a consistent CSS rate of 82–87% at ten years [531,532], and 80–95% for T1/T2 and ISUP GG ≤ 2 PCa [533]. In three studies with data beyond 15 years, the reported CSS rates were 80%, 79% and 58% [531,532,534]. Two studies reported 20-year CSS rates of 57% and 32% [531,534]. The observed heterogeneity in outcomes is due to different inclusion criteria, with some older studies from the pre-PSA era showing worse outcomes [534]. In addition, many patients classified as ISUP GG 1 would now be classified as ISUP GG 2–3 based on the 2005 Gleason classification, suggesting that the above-mentioned results should be considered as minimal and current outcomes would be more favourable. Patients with well-, moderately- and poorly-differentiated tumours had ten-year CSS rates of 91%, 90% and 74%, respectively, correlating with data from a pooled analysis [533]. In screen-detected localised PCa there is also a lead-time bias, resulting in a higher rate of early detected PCa, but also an even higher risk of detecting clinically insignificant PCa that never would have caused any symptoms [530]. Cancer specific survival from untreated screen-detected PCa in patients with ISUP grade groups 1–2 is therefore likely to be even more favourable than for PCa detected of other reasons. Consequently, a high proportion of men with PSA-detected PCa are suitable for conservative management.
The high CSS rate of localised PCa requires that a life expectancy of at least ten years should be considered mandatory for any benefit from curative treatment. Co-morbidity is as important as age in predicting life expectancy. Increasing co-morbidity greatly increases the risk of dying from non-PCa-related causes. In an analysis of 19,639 patients aged > 65 years who were not given curative treatment, most men with a CCI score ≥ 2 had died from competing causes at ten years follow-up regardless of their age at time of diagnosis. Tumour aggressiveness had little impact on OS suggesting that patients could have been spared biopsy and diagnosis of cancer. Men with a CCI score ≤ 1 had a low risk of death at ten years, especially for well- or moderately differentiated lesions [512]. Additionally, in the ProtecT trial (see section 6.2.1.2), prostate cancer-related death was 3% at 15 years compared to death from any cause in 21.7% of patients, numbers that have been further validated in two large population-based studies from Canada and Sweden [535-537].
When managed with non-curative intent, intermediate-risk PCa is associated with ten-year and fifteen-year PCSM rates of 13.0% and 19.6%, respectively [538]. These estimates are based on systematic biopsies and may be overestimated in the era of MRI-targeted biopsies.
The overall evidence indicates that for men with asymptomatic, clinically localised PCa, and with a life expectancy of < 10 years based on co-morbidities and/or age, the oncological advantages of active treatment are unlikely to be relevant to them. Consequently, WW should be adopted for such patients. Estimation of competing benefits of active vs. conservative treatment and death from any cause at ten and fifteen years can be estimated using the PREDICT Prostate tool (https://prostate.predict.nhs.uk/), which is endorsed by the National Institute for Health and Care Excellence in the UK [539]. This highlights the importance of assessing co-morbidity even before considering a biopsy, but also before advising a patient with a PCa diagnosis on the optimal treatment for him.
6.2.1.1. Watchful Waiting
Watchful waiting refers to conservative management for patients deemed unsuitable for curative treatment from the outset and in whom palliative therapy is not yet indicated. The aim of WW is to balance the potential harms and benefits of early hormonal treatment, and patients are clinically ‘watched’ for the development of local or systemic progression with (imminent) disease-related symptoms, at which stage they are then treated palliatively according to their symptoms in order to maintain QoL. Traditionally WW has meant waiting for symptoms of the tumour to develop and has, in some practices, not included regular follow-up in any active way. However, today we have evidence that early hormonal treatment could prolong short term survival (within a few years) for locally advanced disease, for patients with a PSA doubling time (PSA-DT) < 12 months, and for PSA-values over 30-50 ng/mL [540,541]. A more active follow-up of men on WW could therefore be beneficial for the higher risk groups, so that a local or start of metastatic spread progression (often associated with a higher ISUP GG) can be detected before they present with significant symptoms. Hormonal treatment could then be considered before symptoms emerge. The WW strategy should therefore be individualised and planned together with the patient. Biannual PSA, or annual after a period of stable disease, followed by DRE or bone scan if PSA rises significantly, could then be of value.
In a Swedish registry study of men with non-metastatic PCa on WW, after five years 66.2% of patients with low-risk and 36.1% with high-risk disease, and after ten years 25.5% and 10.4% were still alive and not receiving ADT [542]. At ten years, 4.1% and 10.8% had transitioned to castration-resistant disease, respectively. Importantly, 92.3% of low-risk and 84.1% of high-risk patients died due to other causes than PCa after ten years [542].
Watchful waiting vs. radical prostatectomy
There are two RCTs and one Cochrane review comparing the outcomes of WW to radical prostatectomy (RP). The SPCG-4 study was a RCT from the pre-PSA era, randomising patients to either WW or RP in 695 men (24% with nonpalpable disease) [543]. The study found RP to provide superior CSS, OS and progression-free survival (PFS) compared to WW at a median follow-up of 23.6 years (range 3 weeks–28 years). However, the benefit in favour of RP over WW was only apparent after ten years.
The PIVOT trial, a RCT conducted in the early PSA era, made a similar comparison between RP vs. WW in 731 men (50% with nonpalpable disease, 42% low-risk) but in contrast to the SPCG-4, it found little, to no, benefit of RP (cumulative incidence of all-cause death, RP vs. observation: 68% vs. 73%; RR: 0.92, 95% CI: 0.84–1.01) within a median follow-up period of 18.6 years (interquartile range, 16.6 to 20 years) [544]. Exploratory subgroup analysis showed that the borderline benefit from RP was most marked for intermediate-risk disease (RR: 0.84, 95% CI: 0.73–0.98) but there was no benefit in patients with low- or high-risk disease. Overall, no adverse effects on health related QoL (HRQoL) and psychological well-being was apparent in the first five years [545]. However, one of the criticisms of the PIVOT trial is the relatively high overall mortality rate in the WW group compared with more contemporary series, suggesting a selection bias.
A Cochrane review performed a pooled analysis of RCTs comparing RP vs. WW [546]. Three studies were included; the previously mentioned SPCG-4 [543] and PIVOT [544] and the Veteran’s Administration Cooperative Urological Research Group (VACURG) study which was conducted in the pre-PSA era [547]. The authors found that RP compared with WW reduced time to death by any cause (HR: 0.79, 95% CI: 0.70–0.90), time to death by PCa (HR: 0.57, 95% CI: 0.44–0.73) and time to metastatic progression (HR: 0.56, 95% CI: 0.46–0.70) at 29 years’ follow-up. However, RP was associated with higher rates of urinary incontinence (RR: 3.97, 95% CI: 2.34–6.74) and ED (RR: 2.67, 95% CI: 1.63–4.38).
6.2.1.2. Active surveillance
Active surveillance aims to delay or completely avoid unnecessary local curative treatment (surgery/radiation), and consequently unnecessary side effects, in men with low-risk and selected intermediate-risk PCa, and a life expectancy of ten years or more, who do not require immediate treatment. The strategy aims to achieve the correct timing for curative treatment in those who show reclassification during follow-up [548]. Patients remain under close surveillance through structured surveillance programmes with regular follow-up consisting of PSA testing, clinical examination, repeat prostate biopsies, and an increasing role of imaging (usually MRI). Curative treatment is prompted by pre-defined thresholds indicative of development to potentially significant disease, which is still curable, while considering individual life expectancy.
No formal RCT is available comparing AS to curative treatment. Several cohorts have investigated AS in organ-confined disease, the findings of which were summarised in a SR [549,550]. Table 6.2.2 summarises the results of selected AS cohorts. The long-term OS and CSS of patients on AS are very good. However, more than one-third of patients are reclassified during follow-up, most of whom undergo curative treatment due to disease upgrading, increase in disease extent, disease stage, progression, or patient preference. There is variation and heterogeneity between studies regarding exact patient selection, eligibility criteria, and follow-up policies (including frequency of clinical follow-up, use of PSA kinetics, PSA-density, frequency of standard repeat prostate biopsies, frequency and type of imaging such as MRI, and type of biopsy strategy (systematic, MRI-lesion targeted biopsies, combinations, or template biopsies), when active treatment should be instigated (i.e., reclassification criteria), and which outcome measures should be prioritised [548]. For specific guidelines on inclusion criteria and follow-up strategies for AS, see section 6.2.1.2.1.
ProtecT study
ProtecT, randomised 1,643 patients into one of three arms: active treatment with either RP or EBRT or active monitoring (AM) with outcomes reported at ten years and 15 years [535,551]. ProtecT trial did not apply a formal AS strategy. Active monitoring (AM), was a significantly less stringent surveillance strategy, using PSA only, with relaxed criteria to define progression. No repeat biopsies were performed as in AS.
At enrolment sixty-six percent of the patients had low-risk disease, with 90% having a PSA
< 10 ng/mL, 77% ISUP GG 1 (20% ISUP GG 2–3), and 76% had T1c disease. The remaining patients had mainly intermediate-risk disease (approximately 40%).
The key finding was that AM was as effective as active treatment at fifteen years (CSS = 96.9% in the AM-group vs. 97.8% in the RP-group and 97.1% in the EBRT-group, p = 0.53), but an increased metastatic progression risk (9.4% vs. 4.7% and 5.0% respectively), as well as clinical progression at fifteen years (25.9% for AM vs. 10.7% for RP/RT). Death from any cause occurred in 21.7% of the cohort, with similar numbers across treatment groups. Metastases, although rare, were more frequent than seen with comparable AS protocols [549]. A comprehensive characterisation of the ProtecT study cohort was performed after ten years, stratifying patients at baseline according to risk of progression using clinical stage, grade at diagnosis and PSA level [552]. Additionally, detailed clinico-pathological information on participants who received RP were analysed.
The fifteen-year paper reported updated contemporary risk-stratification according to D’Amico (24.1% Intermediate risk, 9.6% high risk), CAPRA (26.4% Score 3-5, 2.5% Score 6-10) and Cambridge Prognostic Group (20.5% Group 2, 8.8% Groups 3-5). Among patients who underwent RP, 50.5% were ISUP GG ≥2, while 28.5% had an increase in pathological stage and 32% had an increase in tumour grade. Additionally, 51% of patients who developed metastases displayed ISUP GG 1 and 47.6% were low CAPRA risk. Over time, 61.1% of patients in the AM group received radical treatment (from 54.8% at ten years). From the ten year report the authors aimed to identify prognostic markers. The results showed that treatment received, age (65–69 vs. 50–64 years), PSA, ISUP GG at diagnosis, cT stage, risk group, number of PCa-involved biopsy cores, maximum length of tumour (median 5.0 vs. 3.0 mm), aggregate length of tumour (median 8.0 vs. 4.0 mm), and presence of perineural invasion were each associated with increased risk of disease progression (p < 0.001 for each). However, these factors could not reliably predict progression in individuals. Notably, 53% (n = 105) of patients who progressed had biopsy ISUP GG 1 disease, although, conversely, none of the participants who received RP and subsequently progressed had pathological ISUP GG 1 tumours. This discrepancy in progression and metastases rate between the AM arm of the ProtecT study and comparable AS protocols can, most likely, be explained by differences in intensity of surveillance, inadequate sampling by PSA testing and 10-core TRUS-guided biopsies.
Nevertheless, the ProtecT study has reinforced the role of deferred active treatment (i.e., either AS or some form of initial AM) as a feasible alternative to active curative interventions in all patients with low-grade and low-stage disease, as well as for many patients with favourable intermediate risk disease. Beyond fifteen years, no RCT-data are available, as yet, although AS is likely to give more reassurance especially in younger men, based on more accurate risk stratification at recruitment and more stringent criteria regarding follow-up, imaging, repeat biopsy and reclassification. Individual life expectancy must continuously be evaluated before considering any active treatment in low-risk patients and in those with up to ten to fifteen years’ individual life expectancy [552].
6.2.1.2.1. Active surveillance - inclusion criteria
Active surveillance inclusion criteria aim to select cases in which delay caused by the initial expectant management strategy does not lead to additional unfavourable outcomes.
Guidance regarding selection and follow-up criteria for AS is limited by the lack of data from prospective RCTs. As a consequence, the international collaborative DETECTIVE study involving healthcare practitioners and patients developed consensus statements for deferred treatment with curative intent for localised PCa, covering all domains of AS [372], as well as a formal SR on the various AS protocols [553]. The most frequently applied criteria include: ISUP GG 1 (on systematic biopsy), clinical stage cT1c or cT2a, PSA < 10 ng/mL and PSA-D < 0.15 ng/mL/cc [549,554]. The latter threshold remains controversial [554,555]. These criteria were supported by the DETECTIVE study consensus. There was no agreement on the maximum number of systematic cores that can be involved with cancer or the maximum percentage core involvement (CI), although there was recognition that extensive disease on MRI should exclude men from AS, even though there is no firm definition on this, especially when targeted biopsies confirm ISUP GG 1 [372]. Magnetic resonance imaging index lesions diameter may provide additional guidance, as thresholds of > 10mm and > 20mm have been used to predict BCR after RP, but not yet used in AS criteria [556]. The Movember consensus group, consisting of 27 healthcare professional and 12 lived experience participants from across the world, agreed that ISUP GG and MRI were the most important criteria for determining eligibility to AS [557].
A SR and meta-analysis found three clinico-pathological variables which were significantly associated with reclassification, high PSA-D, > 2 positive cores (on systematic biopsies), and African-American descent [558]. A review on the risk of progression for African-American men on AS also indicated a potential increased risk of progression, but the association was not strong enough to discourage African-American men from undergoing AS, but thorough confirmatory testing is important [559].
In addition, a previous pathology consensus group suggested excluding men from AS when any of the following features were present: cribriform histology, predominant ductal carcinoma (including pure IDC), sarcomatoid carcinoma, small cell carcinoma, EPE or LVI in needle biopsy [560], or PNI [561].
In men eligible for AS based upon systematic biopsy findings alone who did not have a pre-biopsy MRI, a re-biopsy within six to twelve months (usually referred to as ‘confirmatory biopsy’) is mandatory to exclude sampling error.
6.2.1.2.2. Active surveillance – inclusion of intermediate risk disease
In the ProtecT trial, where 34% of the randomised patients had a D’Amico intermediate- or high-risk disease, there was no statistically significant difference in CSS at 15 years [535].
The outcomes of AS in intermediate-risk PCa has also been analysed in three SRs and meta-analyses, summarising available data on its oncological outcomes and comparing patients with intermediate-risk PCa to patients with low-risk disease [562-564]. The definition of AS was not strictly defined in either of the reviews: instead, the search strategies included ‘active surveillance’ as a search term, and no a priori study protocol was available. The primary outcome was the proportion of patients who remained on AS, whilst secondary outcomes included CSS, OS, and MFS in all three studies.
In the first review seventeen studies were included, incorporating 6,591 patients with intermediate risk disease. Sixteen studies included patients with low- and intermediate-risk disease, hence enabling comparative outcome assessment via pooled analysis. Only one study performed MRI at recruitment and during AS. There was significant clinical heterogeneity in terms of inclusion criteria for intermediate-risk disease. The results showed the proportion of patients who remained on AS was comparable between the low- and intermediate-risk groups after ten- and fifteen-years’ follow-up (OR: 0.97; 95% CI: 0.83–1.14; and OR: 0.86; 95% CI: 0.65–1.13, respectively). Cancer-specific survival was worse in the intermediate-risk group after ten years (OR: 0.47; 95% CI: 0.31–0.69) and fifteen years (OR: 0.34; 95% CI: 0.2–0.58), although it remains unclear whether this is due to less favourable baseline characteristics or due to the delay caused by the initial period of AS. Overall survival was not statistically significantly different at five years’ follow-up (OR: 0.84; 95% CI: 0.45–1.57) but was significantly worse in the intermediate-risk group after ten years (OR: 0.43; 95% CI: 0.35–0.53). Metastases-free survival did not significantly differ after five years (OR: 0.55; 95% CI: 0.2–1.53) but was worse in the intermediate-risk group after ten years (OR: 0.46; 95% CI: 0.28–0.77) [564].
The second review, including 25 studies and a total of 29,673 low- or intermediate-risk patients, showed similar results in terms of treatment-free survival at ten years (RR: 1.16, 95% CI: 0.99-1.36), risk of developing metastases (RR: 5.79, 95% CI: 4.61-7.29), risk of dying from PCa (RR: 3.93, 95% CI: 2.93-5.27), and risk of dying from any cause (RR: 1.44, 95% CI: 1.11-1.86) [562]. In a subgroup analysis of four studies comparing outcomes of patients with intermediate- and low-risk PCa of ISUP GG ≤ 2 (n = 1,900) no statistically significant difference could be found in terms of treatment free survival or risk of developing metastases (RR: 1.03, 95% CI: 0.62-1.71 and RR: 2.09, 95% CI: 0.75-5.82, respectively).
The third, most recent, review included 25 studies of which thirteen studies provided data on treatment free survival, six on CSS and seven on OS. Treatment free survival was not statistically significantly different in the intermediate risk group after five (RR: 0.92, 95% CI: 0.82-1.02), ten (RR: 0.83, 95% CI: 0.55-1.23) or fifteen years (RR: 0.54, 95% CI: 0.21-1.39). Cancer-specific survival was significantly lower after 15 years (RR: 0.92, 95% CI: 0.89-0.96) and OS was significantly lower after ten years (RR: 0.87, 95% CI: 0.82-0.93) in the intermediate risk group. It should be noted that many of the studies included patients with ISUP GG 3 disease. When these studies were excluded no difference in treatment free, cancer specific or OS could be observed [563].
The reviews indicate that AS in unselected intermediate-risk patients implies a higher risk of progression over time. It remains unclear whether this difference only reflects the baseline difference in outcome, that can also be seen when comparing immediate treatment of low- and intermediate-risk PCa, or if the delay in treatment caused any worsening of the outcomes in the intermediate-risk group in any way. All three reviews conclude that AS could be offered to patients with intermediate-risk disease, but they should be informed of a higher risk of progression and the latter two reviews suggests limiting the inclusion of intermediate-risk patients to those with low-volume ISUP GG 2 disease.
The safety of delayed definitive therapy in men with grade reclassification during AS was confirmed in a study comparing 979 patients who underwent immediate RP after diagnosis of ISUP GG 2, 190 who underwent RP within 12 months of upgrading to ISUP GG 2 on AS, and 90 men who underwent RP >12 months after upgrading to ISUP GG 2. Significant predictors of recurrence in multivariable analysis included percentage positive biopsy cores and PSA, but not timing of RP [565].
A Canadian consensus group proposes that low volume ISUP GG 2 (< 10% Gleason pattern 4 on systematic biopsies) may also be considered for AS. These recommendations have been endorsed by the ASCO [245] and the DETECTIVE study consensus [372] for those patients with a PSA < 10 ng/mL and low core positivity. The DETECTIVE study concluded that men with favourable ISUP GG 2 PCa (PSA < 10 ng/mL, low PSA density, clinical stage ≤ cT2a and a low number of positive systematic cores) should also be considered for deferred treatment [372]. In this setting, re-biopsy within six to twelve months to exclude sampling error is even more relevant than in low-risk disease [554,566]. The DETECTIVE study-related qualitative SR aimed to determine appropriate criteria for inclusion of intermediate-risk disease into AS protocols [553]. Out of 371 AS protocols included in the review, more than 50% included patients with intermediate-risk disease on the basis of PSA up to 20 ng/mL (25.3%), ISUP GG 2 or 3 (27.7%), clinical stage cT2b/c (41.6%) and/or direct use of D’Amico risk grouping of intermediate risk or above (51.1%). The DETECTIVE study reached consensus that patients with ISUP GG 3, or patients with intraductal or cribriform histology, should not be considered for AS. The presence of any grade 4 pattern is associated with a 3-fold increased risk of metastases compared to ISUP GG 1, while a PSA up to 20 ng/mL might be an acceptable threshold [566-568], especially in the context of low PSA-D.
The indicator of the tumour volume may be either the number of positive cores, and the length of cancer in each core, based on systematic biopsies, or the volume of the dominant lesion seen on mpMRI [372]. If targeted biopsies based upon mpMRI images are performed, the number of positive cores of the targeted biopsies are not an indicator of the extent of disease or tumour volume when considering a patient for AS due to the altered biopsy protocol.
MRI-targeted biopsies have been associated with up-grading of tumours but improved outcomes [103].
The large prospective PRIAS study on AS expanded inclusion criteria when MRI and targeted systematic biopsies are used at inclusion (https://prias-project.org/modules/articles/article.php?id=1):
- cT ≤ 2
- ISUP: GG 1 or GG 2 without invasive cribriform growth and intraductal carcinoma
- PSA: ≤ 20 ng/mL
- PSA-density: < 0.25 ng/mL/cc
- Number of positive cores
- For ISUP GG 1: No limit.
- For ISUP GG 2 (without invasive cribriform growth and intraductal carcinoma): ≤ 50% systematic cores (where multiple positive cores from the same lesion on MRI count for one positive core).
During follow-up, upgrading is the only criterium for discontinuation, defined as ISUP GG ≥ 3 or ISUP GG ≥ 2 with cribriform growth or intraductal carcinoma, or ISUP GG ≥ 2 with > 50% positive cores.
A multi-disciplinary consensus conference on germline testing has suggested a genetic implementation framework for the management of PCa [165]. Based on consensus, BRCA2-gene testing was recommended for AS discussions and could be performed in men with family history of prostate, breast or ovarian cancers. However, the nature of such discussions and how a positive result influences management were beyond the scope of the project. Currently, BRCA2 mutation does not exclude a patient from AS if tumour factors are otherwise favourable. Furthermore, if included in AS programmes, patients with a known BRCA2 mutation should be cautiously monitored until such time that more robust data are available.
6.2.1.2.3. Tissue-based prognostic biomarker testing for selection for active surveillance
Biomarkers, including Oncotype Dx®, Prolaris®, Decipher®, PORTOS and ProMark® are promising; however, further data and comparisons with other parameters (including MRI) will be needed before such markers can be used in standard clinical practice [240].
6.2.1.2.4. Magnetic resonance imaging for selection for active surveillance
Two RCTs and a SR, showed that adding MRI-targeted biopsy to systematic sampling at confirmatory biopsy increased the number of cancers labelled ISUP GG ≥ 2 and thus may aid patient selection for AS, although the impact of MRI and targeted biopsies with corresponding stage shift on long-term oncological outcomes of AS is lacking [126,569-574]. Adding MRI-targeted biopsy to systematic sampling at confirmatory biopsy improved upgrade detection by increments of 0-7.9 per 100 men depending on the series [569]. In a meta-analysis of 6 studies, the rate of upgrading to ISUP GG ≥ 2 cancer increased from 20% (95% CI: 16–25%) to 27% (95% CI: 22–34%) when MRI-targeted biopsy was added to systematic biopsy [574]. The Active Surveillance MRI Study (ASIST) randomised men on AS scheduled for confirmatory biopsy to either 12-core systematic biopsy or to MRI with targeted biopsy (when indicated), combined with systematic biopsy (up to 12 cores in total). After two years of follow-up, use of MRI before confirmatory biopsy resulted in fewer failures of surveillance (19% vs. 35%, p = 0.017) and in fewer patients progressing to ISUP GG ≥ 2 cancer (9.9% vs. 23%, p = 0.048) [572]. However, systematic biopsy retains its additional value, which argues for a combined biopsy approach [569,574]. The DETECTIVE study agreed that men eligible for AS after combined systematic- and MRI-targeted biopsy do not require a confirmatory biopsy, a recommendation further supported by the results of the MRIAS trial [372,575].
If the PCa diagnosis is made on MRI-targeted biopsy alone in order to lower the risk of over detection of insignificant (see section 5.4.1 and 5.4.2), and the number of positive systematic cores used as an indication for tumour volume during AS is not available, MRI lesion diameter may be used as a surrogate, although specific definitions have not yet been tested in an AS setting (e.g. for ISUP GG 2 tumours no PIRADS 5 or < 20 mm lesion size) [556].
A few studies indicate that PSMA-PET-CT or PSMA-PET-MRI may have additional value to above mentioned clinico-pathological variables for risk stratification before AS [127,576]. However, so far, the studies are too small, the follow-up too short, and association with long-term oncological outcomes is lacking, to draw any hard conclusions and for this modality to be recommended outside clinical trials.
6.2.1.2.5. Active surveillance follow-up
Based on the DETECTIVE consensus study, the surveillance strategy should be based on serial DRE (at least once yearly), PSA (at least once, every six months), and repeated biopsy (no consensus on frequency, but 1-4-7 years is an often-applied schedule).
A panel SR incorporating 263 surveillance protocols showed that 78.7% of protocols mandated per-protocol repeat biopsies within the first two years and that 57.7% of the protocols performed repeat biopsy at least every three years for ten years after the start of AS [553].
There was clear agreement in the DETECTIVE consensus meeting as well as in the Movember consensus group that a PSA change alone, including PSA-doubling time (PSA-DT, < 3 years) should not change management based on its weak link with grade progression [577,578] but rather trigger further investigation such as biopsy or repeat-MRI. It was also agreed that changes on repeat MRI during AS needed a repeat biopsy before considering continuing to active treatment [372,557].
The Movember consensus group made a number of recommendations that in some ways differ from the DETECTIVE consensus study, e.g. routine DRE was not supported if MRI or other imaging was carried out routinely during AS, if MRI combined with other parameters (PSA kinetics and density) are stable routine biopsy may be omitted, and change in clinical parameters should prompt MRI with possible biopsy rather than immediate biopsy [557].
6.2.1.2.6. Magnetic resonance imaging for follow-up during active surveillance
The Prostate Cancer Radiological Estimation of Change in Sequential Evaluation (PRECISE) criteria were established to standardise the assessment of tumour progression on serial MRI [579]. PRECISE is a strong predictor of histological upgrading [580,581]. Two independent meta-analyses assessed the value of MRI progression criteria for predicting histological progression (mostly defined as progression to ISUP GG ≥ 2). The pooled histological progression rate was 27% in both reviews. If biopsies were triggered only by MRI progression findings, approximately two thirds of the biopsies would be avoided, at the cost of missing 40% of men with histological progression. In addition, at least half of biopsied men would have had negative findings for histological progression and thus would have undergone unnecessary biopsies. If histological progression was restricted to progression to ISUP GG > 3, approximately 30% of histological progression would be missed and approximately 80% of the biopsies performed would be unnecessary. The use of the PRECISE criteria did not seem to change these results [582,583]. This supports maintaining protocol-mandated repeat biopsies during the course of AS.
Another study analysed a prospectively-maintained AS cohort of 369 patients (272 with ISUP GG 1 cancer and 97 with ISUP GG 2 cancer) who had been selected for AS after combined systematic and MRI-targeted sampling during confirmatory biopy [584]. At two years, systematic biopsy, MRI-targeted biopsy and combined biopsy detected grade progression in 44 (15.9%), 73 (26.4%) and 90 patients (32.5%), respectively. This suggests that both biopsy approaches retain added value, not only for confirmatory biopsy, but also during AS [584]. Systemtic biopsy cores may thus be considered to be added to follow-up biopsy to rule out more widespread disease [208,210,314]. The disadvantage of overdiagnosis due to systematic cores is not present in the AS follow-up setting. On the other hand, extra biopsy cores may cause discomfort and, as in the primary diagnostic setting, the risk of leaving significant PCa undetected is small, and of limited relevance in a surveillance setting. As in the primary setting, the strategy of targeted/perilesional cores is therefore also recommended during AS repeat biopsy.
6.2.1.2.7. Individualised repeat biopsy during active surveillance
The basis for AS protocols includes standard repeat biopsy. However, several factors have been found to be associated with low re-classification rates and long PFS and can be used to individualise the need and frequency of AS biopsy schedules: low PSA-D [575,585-587], low PSA velocity (PSAV) [588,589], negative biopsy (i.e., no cancer at all) at confirmatory or repeat biopsy during AS [521], and negative baseline or repeat MRI during AS [575,585-587,590-593]. Negative repeat biopsy during AS was associated with a 50% decrease in the risk of future reclassification and upgrading [594]. In a single-centre AS cohort of 514 patients who underwent at least three protocol-mandated biopsies after diagnosis (the confirmatory biopsy and at least two additional surveillance biopsies), men with one negative biopsy (i.e., no cancer at all) at confirmatory or second biopsy, or men with two consecutive negative biopsies had a lower likelihood of a positive third biopsy and significantly better 10-year treatment free survival [595]. Patients with stable (PRECISE 3) on repeat MRI during AS combined with a low PSA-D (<0.15) have a very low rate of progression and may be a group in whom standard repeat biopsy may be omitted [596].
6.2.1.2.8. Active Surveillance - change in treatment
Men may remain on AS whilst they have a life expectancy of > 10 years and the disease remains insignificant. A transition from AS to WW due to rising age or new comorbidity should be incorporated within conservative management strategies for PCa and in discussion with patients [597].
Histopathology criteria are the strongest reason to trigger a change in management, including. reclassification to ISUP GG 3 or detection of cribriform or intraductal growth patterns, based on systematic biopsy. The exact criteria in the targeted biopsy era remain debated. MRI-targeted biopsy induces a grade shift and ISUP GG 2–3 cancers detected by MRI-targeted biopsy have, on average, a better prognosis than those detected by systematic sampling. Also, men upgraded during AS, have more favourable outcomes as men with the same ISUP GG detected at first biopsy [598]. As an increasing number of men with favourable intermediate-risk disease are managed with AS (see section 6.2.1.2), progression to ISUP GG 2 should not be used a hard reason to stop AS, especially when found on targeted biopsy. In addition, as acknowledged in the DETECTIVE consensus meeting, the number of positive cores is not an indicator of tumour volume anymore if targeted biopsies are performed [372,599]. Based on the findings of a SR incorporating 271 reclassification protocols, patients with low-volume ISUP GG 2 disease at recruitment, and with increased systematic core positivity (> 3 cores involvement [> 50% per core]) on repeat systematic biopsies not using MRI, should be reclassified [553]. As for inclusion, MRI tumour volume may be used during follow-up as a surrogate for tumour volume estimation based on systematic biopsies, though specific definitions are lacking. Furthermore, in a study from the MUSIC registry over half of men with favourable intermediate-risk PCa on AS remained free of treatment five years after diagnosis [600]. Their results are in concordance with the DETECTIVE and the Movember consensus statements and indicate that most men on AS will not lose their window of cure and have similar short-term oncologic outcomes as men undergoing up-front treatment and that AS is an oncologically safe option for appropriately selected men with favourable intermediate-risk PCa.
6.2.1.2.9. Psychological factors during active surveillance
Anxiety about continued surveillance occurs in around 10% of patients on AS [601] and was recognised as a valid reason for active treatment [369]. An alternative for patients suitable for continuing AS would be to offer psychological support to reduce the level of anxiety, as also stated by the Movember consensus group [557]. A review on patient reported factor influencing the decision making, including thirteen qualitative papers and 426 men, identified several factors influencing the decision making when considering AS. Among the identified factors were personal risk assessment, influence of family and friends, beliefs about treatment as well as doctor and system factors, underscoring the importance of individualised, relevant, and clear information to support decision making [602]. A population-based cohort study from Sweden on regional differences in AS uptake and subsequent transition to radical treatment concluded that a regional tradition of a high uptake of AS was associated with a lower probability of transition to radical treatment, but not with AS failure [603]. These studies further emphasise the importance of thorough information and discussion with the patients on pros/cons of AS versus active treatment already at the time of diagnosis for the patients to feel secure in their treatment choice and to avoid over-treatment.
6.2.1.2.10. Interventions during active surveillance
A review on potential interventions during AS found that use of 5-ARIs was associated with improved progression-free survival (PFS; hazard ratio: 0.59; 95% confidence interval 0.48-0.72), with limited increased toxicity [604].
A phase II RCT randomised patients to AS plus enzalutamide or AS alone. This study indicated that PSA progression could be delayed, and the odds of a negative biopsy increased during the median follow-up time of 1.3 years, but patients had more side effects of the treatment without showing any long-term benefits of the treatment [605].
Table 6.2.2 Active surveillance oncological outcomes in large cohorts with longer-term follow-up
| Studies | N | Median FU (mo) | 10-year OS (%) | 10-year CSS (%) |
| Adamy, et al. 2011 [551] | 533-1,000 | 48 | 90 | 99 |
| Godtman, et al. 2013 [554] | 439 | 72 | 81 | 99.5 |
| Klotz, et al. 2015 [555] | 993 | 77 | 85 | 98.1 |
| Tosoian, et al. 2020 [557] | 1,818 | 60 | 93 | 99.9 |
| Carlsson, et al. 2020 [558] | 2,664 | 52 | 94 | 100 |
| Newcomb, et al. 2024 [606] | 2,155 | 86 | 95 | 99.9 |
CSS = cancer-specific survival; FU = follow-up; mo = months; N = number of patients; OS = overall survival; RP = radical prostatectomy.
6.2.1.3. Summary of evidence and recommendations for active surveillance strategy
| Summary of evidence | LE |
| The AS strategy should be based on PSA (at least once every six months), serial DRE (at least once yearly) and repeated biopsy. Serial DRE may be omitted if MRI is stable. | 3 |
| Magnetic resonance imaging detects more cancers labelled with higher ISUP GG and may be used before starting AS (if not performed earlier), although impact on long-term oncological endpoints is lacking. | |
| Serial DRE may be omitted if MRI is stable. | |
| A progression on MRI mandates a repeat biopsy, to confirm histological progression, before a change in treatment strategy. | |
| A stable MRI (PRECISE 1-3) does not make repeat biopsy superfluous, but in patients with low-risk tumour and a stable low PSA-D < 0.15 may be excluded. | |
| No modality has shown superiority over any other active management options or deferred active treatment in terms of overall- and PCa-specific survival for clinically localised low/intermediate-risk disease. | 2 |
| Recommendations | Strength rating |
| Offer active surveillance (AS) as standard of care for low-risk disease. | Strong |
| Exclude patients with cribriform or intraductal histology on biopsy from AS. | Strong |
| Perform magnetic resonance imaging (MRI) before a confirmatory biopsy if no MRI has been performed before the initial biopsy. | Strong |
| Take targeted and perilesional biopsy cores (of any PI-RADS ≥ 3 lesion) if a confirmatory or repeat biopsy is performed. | Strong |
| Perform per-protocol confirmatory prostate biopsies if MRI is not available. | Weak |
| Do not perform confirmatory biopsies if a patient has had upfront MRI and targeted biopsies. | Weak |
| Base the strategy of AS on a strict follow-up protocol including PSA (at least once every six months), digital rectal examination (DRE) (at least once yearly), and repeated biopsy (every 2-3 years for 10 years). | Strong |
| Exclude patients with a low-risk PCa, a stable MRI (PRECISE 3) and a stable low PSA density (< 0.15) from repeat biopsy when MRI is repeated before repeat biopsy. In addition, serial DRE may be omitted if MRI is stable. | Weak |
| Perform MRI and repeat biopsy if PSA is rising (PSA-doubling time < 3 years). | Strong |
| Base change in treatment on biopsy progression, not on progression on MRI, PSA, and/or DRE. | Weak |
6.2.2. Radical prostatectomy
6.2.2.1. Introduction
The goal of RP by any approach is the eradication of cancer while, whenever possible, preserving pelvic organ function [607]. The procedure involves removing the entire prostate with its capsule intact and SVs, followed by vesico-urethral anastomosis. Surgical approaches have expanded from perineal and retropubic open approaches to laparoscopic and robotic-assisted techniques; anastomoses have evolved from Vest approximation sutures to continuous suture watertight anastomoses under direct vision and mapping of the anatomy of the dorsal venous complex (DVC) and cavernous nerves has led to excellent visualisation and potential for preservation of erectile function [608]. The main results from multi-centre RCTs involving RP are summarised in Table 6.2.3.
Table 6.2.3: Oncological results of radical prostatectomy in organ-confined disease in RCTs
| Study | Acronym | Population | Treatment period | Median FU (mo) | Risk category | CSS (%) |
| Bill-Axelson, et al. 2018 [543] | SPCG-4 | Pre-PSA era | 1989-1999 | 283 | Low risk & intermediate risk | 80.4 (at 23 yr.) |
Wilt, et al. 2017 [544] | PIVOT | Early years of PSA testing | 1994-2002 | 152 | Low risk & intermediate risk | 95.9 91.5 (at 19.5 yr.) |
Hamdy, et al. 2023 [535] | ProtecT | Screened population | 1999-2009 | 180 | Mainly low- & intermediate risk | 97 (at 15 yr.) |
CSS = cancer-specific survival; FU = follow-up; mo = months; PSA = prostate-specific antigen; yr. = year.
6.2.2.2. Pre-operative preparation
6.2.2.2.1. Pre-operative patient education
As before any surgery appropriate education and patient consent is mandatory prior to RP. Peri-operative education has been shown to improve long-term patient satisfaction following RP [609]. Augmentation of standard verbal and written educational materials such as use of interactive multimedia tools [610,611] and pre-operative patient-specific 3D printed prostate models has been shown to improve patient understanding and satisfaction and should be considered to optimise patient-centred care [612].
Additional consideration should be given to patients who have undergone prior transurethral resection of the prostate (TURP). According to SR and meta-analysis of non-randomised studies, prior TURP can prolong operative and catheter time, have higher complications, require more bladder neck reconstruction and less nerve sparing resulting in higher positive margin rate (RR 1.24, p = 0.03), higher incontinence (RR 1.24, p = 0.03) and erectile function (RR 0.8, p < 0.001) at 12 months after RARP [613]. While patients with prior TURP are typically older, which is also a predictor for these outcomes in RARP patients, prior TURP is worthy of consideration in pre-operative counselling.
6.2.2.3. Surgical techniques
6.2.2.3.1. Prostatic anterior fat pad dissection and histologic analysis
Several multi-centre and large single-centre series have shown the presence of lymphoid tissue within the fat pad anterior to the endopelvic fascia; the prostatic anterior fat pad (PAFP) [614-620]. This lymphoid tissue is present in 5.5–10.6% of cases and contains metastatic PCa in up to 1.3% of intermediate- and high-risk patients.
When positive, the PAFP is often the only site of LN metastasis. The PAFP is therefore a rare but recognised route of spread of disease. The PAFP is always removed at RP for exposure of the endopelvic fascia and should be sent for histologic analysis as per all removed tissue.
6.2.2.3.2. Management of the dorsal venous complex
Since the description of the anatomical open RP by Walsh and Donker in the 1980s, various methods of controlling bleeding from the DVC have been proposed to optimise visualisation [621].
In the open setting, blood loss and transfusion rates have been found to be significantly reduced when ligating the DVC prior to transection [622]. However, concerns have been raised regarding the effect of prior DVC ligation on apical margin positivity and continence recovery due to the proximity of the DVC to both the prostatic apex and the urethral sphincter muscle fibres.
In the robotic-assisted laparoscopic technique, due to the increased pressure of pneumoperitoneum, whether prior DVC ligation was used or not, blood loss was not found to be significantly different in one study [623]. In another study, mean blood loss was significantly less with prior DVC ligation (184 vs. 176 mL, p = 0.033), however it is debatable whether this was clinically significant [624]. The positive apical margin rate was not different, however, the latter study showed earlier return to full continence at five months post-operatively in the no prior DVC ligation group (61% vs. 40%, p < 0.01). Ligation of the DVC can be performed with standard suture or using a vascular stapler. One study found significantly reduced blood loss (494 mL vs. 288 mL) and improved apical margin status (13% vs. 2%) when using the stapler [625].
Given the relatively small differences in outcomes, the surgeon’s choice to ligate prior to transection or not, or whether to use sutures or a stapler, will depend on their familiarity with the technique and the equipment available.
6.2.2.3.3. Nerve-sparing surgery
During prostatectomy, preservation of the neurovascular bundles (NVB) with parasympathetic nerve branches of the pelvic plexus can spare erectile function [626,627].
Although age and pre-operative function may remain the most important predictors for post-operative erectile function, NS has also been associated with improved continence outcomes and may therefore still be relevant for men with poor erectile function [628,629]. A large SR and meta-analysis reported that bilateral NS resulted in improved urinary continence recovery (RR 1.08 at 12 months, p < 0.0001) across all time points with heterogeneous pooled estimates [630]. The association with continence may be mainly due to the dissection technique used during NS surgery, and not due to the preservation of the NVB themselves [628].
Extra-, inter-, and intra-fascial dissection planes can be planned, with those closer to the prostate and performed bilaterally associated with superior (early) functional outcomes [631-634]. Furthermore, many different techniques are propagated such as retrograde approach after anterior release (vs. antegrade), and athermal and traction-free handling of bundles [635-637]. Nerve-sparing (NS) surgery may be performed using clips or low bipolar energy without clear benefit favouring one technique over another regarding functional outcomes [638].
Patient selection for nerve sparing remains challenging for clinicians. A 2021 SR of nineteen studies analysing the parameters used for selection of NS found that individual clinical and radiological factors were poor at predicting EPE, and consequently, the appropriateness of NS. However, nomograms that incorporated mpMRI performed better [639]. High-risk patients can be considered, as a large retrospective study prone to selection bias for NS reported that NS did not affect BCR, risk of metastasis or of death regardless of stage or ISUP GG [640].
A reasonable concern is the oncological compromise and positive surgical margin rate. A 2022 SR of 18 comparative studies (no RCTs) of NS vs. non-nerve-sparing RP showed a RR of side-specific positive margins of 1.5, but none of them included patients with high-risk PCa [641]. There was no effect seen of NS on BCR. However, follow-up was short, and studies were subject to selection bias with mainly low-risk patients. For those patients with high-risk PCa, side-specific NS was avoided if disease was palpable or EPE was present on MRI. Indeed, a 2019 SR showed that MRI affected the decision to perform NS or not in 35% of cases without any negative impact on surgical margin rate [642].
In summary, the quality of data is not adequate to permit a strong recommendation in favour of NS or non-nerve-sparing, but pre-operative risk factors for side-specific EPE such as PSA, PSA density, clinical stage, ISUP grade group, and PIRADS score, EPE and capsule contact length on MRI, should be taken into account.
6.2.2.3.4. Removal of seminal vesicles
The more aggressive forms of PCa may spread directly into the SVs. For oncological clearance, the SVs have traditionally been removed intact with the prostate specimen [643]. However, in some patients the tips of the SVs can be challenging to dissect free. Furthermore, the cavernous nerves run past the SV tips such that indiscriminate dissection of the SV tips could potentially lead to ED [644]. However, a RCT comparing nerve-sparing RP with and without a SV-sparing approach found no difference in margin status, PSA recurrence, continence or erectile function outcomes. Whilst complete SV removal should be the default, preservation of the SV tips may be considered in cases of low risk of involvement.
6.2.2.3.5. Bladder neck management
Bladder neck mucosal eversion
Some surgeons perform mucosal eversion of the bladder neck as its own step in open RP with the aim of securing a mucosa-to-mucosa vesico-urethral anastomosis and avoiding anastomotic stricture. Whilst bringing bladder and urethral mucosa together by the everted bladder mucosa covering the bladder muscle layer, this step may actually delay healing of the muscle layers. An alternative is to simply ensure bladder mucosa is included in the full thickness anastomotic sutures. A non-randomised study of 211 patients with and without bladder neck mucosal eversion showed no significant difference in anastomotic stricture rate [645]. The strongest predictor of anastomotic stricture in RP is current cigarette smoking [646], but it is also 2.2 higher in open RP than RARP [647].
Bladder neck preservation
Whilst the majority of urinary continence is maintained by the external urethral sphincter at the membranous urethra (see below), a minor component is contributed by the internal lissosphincter at the bladder neck [648]. Preservation of the bladder neck has therefore been proposed to improve continence recovery post-RP. A RCT assessing continence recovery at twelve months and four years showed improved objective and subjective urinary continence in both the short- and long term without any adverse effect on oncological outcome [649]. These findings were confirmed by a SR [650]. However, concern remains regarding margin status for cancers located at the prostate base.
A SR addressing site-specific margin status found a mean base-specific positive margin rate of 4.9% with bladder neck preservation vs. only 1.9% without [648]. This study was inconclusive, but it would be sensible to exercise caution when considering bladder neck preservation if significant cancer is known to be at the prostate base. Bladder neck preservation should be performed routinely when the cancer is distant from the base. However, bladder neck preservation cannot be performed in the presence of a large median lobe or a previous transurethral resection of the prostate (TURP) [651].
6.2.2.3.6. Urethral length preservation
The membranous urethra sits immediately distal to the prostatic apex and is chiefly responsible, along with its surrounding pelvic floor support structures, for urinary continence. It consists of the external rhabdosphincter which surrounds an inner layer of smooth muscle. Using pre-operative MRI, the length of membranous urethra has been shown to vary widely.
Systematic reviews and meta-analyses found that every extra millimetre of membranous urethral length seen on MRI pre-operatively improves early return to continence post-RP [652-654]. A greater membranous urethral length as measured on preoperative MRI was an independent prognostic factor for return to urinary continence within one month after RP and remained prognostic at twelve months [654]. Therefore, it is likely that preservation of as much urethral length as possible during RP will maximise the chance of early return to continence. It may also be useful to measure urethral length pre-operatively on MRI to facilitate counselling of patients on their relative likelihood of early post-operative continence [655].
6.2.2.3.7. Techniques of vesico-urethral anastomosis
Following prostate removal, the bladder neck is anastomosed to the membranous urethra. The objective is to create a precisely aligned, watertight, tension-free, and stricture-free anastomosis that preserves the integrity of the intrinsic sphincter mechanism. Several methods have been described, based on the direct or indirect approach, the type of suture (i.e. barbed vs. non-barbed/monofilament), and variation in suturing technique (e.g., continuous vs. interrupted, or single-needle vs. double-needle running suture). The direct vesico-urethral anastomosis, which involves the construction of a primary end-to-end inter-mucosal anastomosis of the bladder neck to the membranous urethra by using 6 interrupted sutures placed circumferentially, has become the standard method of reconstruction for open RP [656].
The development of laparoscopic- and robotic-assisted techniques to perform RP have facilitated the introduction of new suturing techniques for the anastomosis. A SR and meta-analysis compared unidirectional barbed suture vs. conventional non-barbed suture for vesico-urethral anastomosis during robotic-assisted radical prostatectomy (RARP) [657]. The review included three RCTs and found significantly reduced anastomosis time, operative time and posterior reconstruction time in favour of the unidirectional barbed suture technique, but there were no differences in post-operative leak rate, length of catheterisation and continence rate. However, no definitive conclusions could be drawn due to the relatively low quality of the data. In regard to suturing technique, a SR and meta-analysis compared continuous vs. interrupted suturing for vesico-urethral anastomosis during RP [658]. The study included only one RCT with 60 patients [659]. Although the review found slight advantages for continuous suturing over interrupted suturing in terms of catheterisation time, anastomosis time and rate of extravasation, the overall quality of evidence was low and no clear recommendations were possible. A RCT [660] compared the technique of suturing using a single absorbable running suture vs. a double-needle single-knot running suture (i.e. Van Velthoven technique) in laparoscopic RP [661]. The study found slightly reduced anastomosis time with the single running suture technique, but anastomotic leak, stricture, and continence rates were similar.
Overall, although there are a variety of approaches, methods, and techniques for performing the vesico-urethral anastomosis, no clear recommendations are possible due to the lack of high-certainty evidence. In practice, the chosen method should be based on surgeon experience and individual preference [656-661].
6.2.2.3.8. Urinary catheter
A urinary catheter is routinely placed during RP to enable bladder rest and drainage of urine while the vesicourethral anastomosis heals. Compared to a traditional catheter duration of around 1 week, some centres remove the transurethral catheter early (post-operative day 2–3), usually after thorough anastomosis with posterior reconstruction or in patients selected peri-operatively on the basis of anastomosis quality [662-665]. No higher complication rates were found. Although shorter catheterisation has been associated with more favourable short-term functional outcomes, no differences in long-term function were found [666]. One RCT has shown no difference in rate of UTI following indwelling catheter (IDC) removal whether prophylactic ciprofloxacin was given prior to IDC removal or not, suggesting antibiotics should not be given at catheter removal [667].
As an alternative to transurethral catheterisation, suprapubic catheter insertion during RP has been suggested. Some reports suggest less bother regarding post-operative hygiene and pain [668-672], while others did not find any differences [673,674]. No impact on long-term functional outcomes were seen.
6.2.2.3.9. Cystography prior to catheter removal
Cystography may be used prior to catheter removal to check for a substantial anastomotic leak. If such a leak is found, catheter removal may then be deferred to allow further healing and sealing of the anastomosis. However, small comparative studies suggest that a cystogram to assess anastomotic leakage is not indicated as SOC before catheter removal eight to ten days after surgery [675]. If a cystogram is used, men with LUTS, large prostates, previous TURP or bladder neck reconstruction, may benefit as these factors have been associated with leakage [676,677]. Contrast-enhanced transrectal US is an alternative [678].
6.2.2.3.10. Use of a pelvic drain
A pelvic drain has traditionally been used in RP for potential drainage of urine leaking from the vesico-urethral anastomosis, blood, or lymphatic fluid when a PLND has been performed. Two RCTs in the robotic-assisted laparoscopic setting have been performed [679,680]. Patients with urine leak at intra-operative anastomosis watertight testing were excluded. Both trials showed non-inferiority in complication rates when no drain was used. When the anastomosis is found to be watertight intra-operatively, it is reasonable to avoid inserting a pelvic drain. There is no evidence to guide usage of a pelvic drain in PLND.
6.2.2.3.11. Considerations during minimally-invasive radical prostatectomy
Minimally-invasive radical prostatectomy, including LRP and RARP, is being used more commonly due to many factors.
6.2.2.3.11.1. Pneumoperitoneum pressure
Reduced blood loss has been reported with minimally-invasive surgery [681], where use of pneumoperitoneum is likely to be a significant contributing factor. Various pneumoperitoneum pressures are used, with higher pressures associated with less bleeding and more surgical working space at the expense of increased abdominal pressure and associated physiological changes. A randomised triple-blinded study comparing RARP (with standard DVC ligation) low-pressure (7 mmHg) versus standard-pressure (12 mmHg) pneumoperitoneum showed that in 98 patients, low pressure was associated with better post-operative quality of recovery and improved pain (p = 0.001), physical comfort (p = 0.007) and emotional state (p = 0.006) on postoperative day 1 at the expense of statistically higher blood loss of questionable clinical relevance (mean 227 ml vs. 159.9ml;
p = 0.001) [682].
6.2.2.4. Acute and chronic complications of radical prostatectomy
Post-operative incontinence and ED are common problems following surgery for PCa. A key consideration is whether these problems are reduced by using newer techniques such as RARP. Systematic reviews have documented complication rates after RARP [681,683-686], and can be compared with contemporaneous reports after radical retropubic prostatectomy (RRP) [687]. A prospective controlled non-RCT of patients undergoing RP in fourteen centres using RARP or RRP showed that twelve months after RARP, 21.3% of patients were incontinent, as were 20.2% after RRP (adjusted OR: 1.08; 95% CI: 0.87–1.34) [688]. Erectile dysfunction was observed in 70.4% after RARP and 74.7% after RRP. The adjusted OR was 0.81 (95% CI: 0.66–0.98) [688].
A SR and meta-analysis of unplanned hospital visits and re-admissions post-RP analysed 60 studies with over 400,000 patients over a 20-year period up to 2020. It found an emergency room visit rate of 12% and a hospital re-admission rate of 4% at 30 days post-operatively [689].
A RCT comparing RARP and RRP reported outcomes at twelve weeks in 326 patients and functional outcomes at two years [690]. Urinary function scores did not differ significantly between RRP vs. RARP at six and twelve weeks post-surgery (74–50 vs. 71–10, p = 0.09; 83–80 vs. 82–50, p = 0.48), with comparable outcomes for sexual function scores (30–70 vs. 32–70, p = 0.45; 35–00 vs. 38–90, p = 0.18). In the RRP group fourteen (9%) patients had post-operative complications vs. six (4%) in the RARP group. The intra- and peri-operative complications of RRP and RARP are listed in Table 6.1.4. Table 6.1.5 lists the Clavien-Dindo definition of surgical complications. The early use of phosphodiesterase-5 inhibitors (PDE5Is) in penile rehabilitation remains controversial resulting in a lack of clear recommendations.
A subsequent meta-analysis of five RCTs (1,205 patients) that compared RARP with LRP showed no difference in continence at twelve months (OR 1.95, 95% CI 0.67 – 5.62) or oncological outcomes (positive margin rate, biochemical recurrence); however, RARP resulted in better 3- (OR 1.81) and 6-month (OR 1.88) continence outcomes as well as erectile recovery in pre-operatively potent patients (OR 4.05, p = 0.003) [691].
Table 6.2.4: Intra-and peri-operative complications of retropubic RP, laparoscopic RP and RARP (adapted from [681])
| Predicted probability of event | RARP (%) | Laparoscopic RP (%) | RRP (%) |
| Bladder neck contracture | 1.0 | 2.1 | 4.9 |
| Anastomotic leak | 1.0 | 4.4 | 3.3 |
| Infection | 0.8 | 1.1 | 4.8 |
| Organ injury | 0.4 | 2.9 | 0.8 |
| Ileus | 1.1 | 2.4 | 0.3 |
| Deep-vein thrombosis | 0.6 | 0.2 | 1.4 |
| Predicted rates of event | RARP (%) | Laparoscopic RP (%) | RRP (%) |
| Clavien-Dindo I | 2.1 | 4.1 | 4.2 |
| Clavien-Dindo II | 3.9 | 7.2 | 17.5 |
| Clavien-Dindo IIIa | 0.5 | 2.3 | 1.8 |
| Clavien-Dindo IIIb | 0.9 | 3.6 | 2.5 |
| Clavien-Dindo IVa | 0.6 | 0.8 | 2.1 |
| Clavien-Dindo V | < 0.1 | 0.2 | 0.2 |
RALP = robot-assisted laparoscopic prostatectomy; RP = radical prostatectomy; RRP = radical retropubic prostatectomy.
Table 6.2.5: Clavien-Dindo grading of surgical complications [692]
| Grade | Definition |
| I | Any deviation from the normal post-operative course not requiring surgical, endoscopic or radiological intervention. This includes the need for certain drugs (e.g. antiemetics, antipyretics, analgesics, diuretics and electrolytes), treatment with physiotherapy and wound infections that are opened at the bedside |
| II | Complications requiring drug treatments other than those allowed for Grade I complications; this includes blood transfusion and total parenteral nutrition (TPN) |
| IIIa | Complications requiring surgical, endoscopic or radiological intervention - intervention not under general anaesthetic |
| IIIb | Complications requiring surgical, endoscopic or radiological intervention - intervention under general anaesthetic |
| IVa | Life-threatening complications; this includes central nervous system (CNS) complications - single-organ dysfunction (including dialysis) |
| IVb | Life-threatening complications; this includes CNS complications (e.g. brain haemorrhage, ischaemic stroke, subarachnoid haemorrhage) which require intensive care, but excludes transient ischaemic attacks (TIAs) - multi-organ dysfunction |
| V | Death of the patient |
6.2.2.4.1. Effect of anterior and posterior reconstruction on continence
Preservation of integrity of the external urethral sphincter is critical for continence post-RP. Less clear is the effect of reconstruction of surrounding support structures to return to continence. Several small RCTs have been conducted, however, pooling analyses is hampered by variation in the definitions of incontinence and surgical approach, such as open vs. robotic and intra-peritoneal vs. extra-peritoneal. In addition, techniques used to perform both anterior suspension or reconstruction and posterior reconstruction are varied. For example, anterior suspension is performed either through periosteum of the pubis or the combination of ligated DVC and puboprostatic ligaments (PPL). Posterior reconstruction from rhabdosphincter is described to either Denonvilliers fascia posterior to bladder or to posterior bladder wall itself.
Two trials assessing posterior reconstruction in RARP found no significant improvement in return to continence [693,694]. A third trial using posterior bladder wall for reconstruction showed only an earlier return to 1 pad per day (median 18 vs. 30 days, p = 0.024) [695]. When combining both anterior and posterior reconstruction, where for anterior reconstruction the PPL were sutured to the anterior bladder neck, another RCT found no improvement compared to a standard anastomosis with no reconstruction [696].
Four RCTs including anterior suspension have also shown conflicting results. Anterior suspension alone through the pubic periosteum, in the setting of extra-peritoneal RARP, showed no advantage [697]. However, when combined with posterior reconstruction in RRP, one RCT showed significant improvement in return to continence at one month (7.1% vs. 26.5%, p = 0.047) and three months (15.4% vs. 45.2%, p = 0.016), but not at six months (57.9% vs. 65.4%, p = 0.609) [698]. Another anterior plus posterior reconstruction RCT using the Advanced Reconstruction of VesicoUrethral Support (ARVUS) technique and the strict definition of continence of ‘no pads‘, showed statistically significant improvement in continence at 2 weeks (43.8% vs. 11.8%), 4 weeks (62.5% vs. 14.7%), 8 weeks (68.8% vs. 20.6%), six months (75% vs. 44.1%) and twelve months (86.7% vs. 61.3%), when compared to standard posterior Rocco reconstruction [699]. Anterior suspension alone through the DVC and PPL combined without posterior construction in the setting of RARP has shown improvement in continence at one month (20% vs. 53%, p = 0.029), three months (47% vs. 73%, p = 0.034) and six months (83% vs. 100%, p = 0.02), but not at twelve months (97% vs. 100%, p = 0.313) [700]. Together, these results suggest a possible earlier return to continence, but no long-term difference.
A novel method of urethral reconstruction with peritoneal support flaps was shown in a randomised trial compared to standard RARP (n = 96) to improve urinary continence recovery (0-1 pad) at 1-month (73% vs. 49%, p = 0.017) and 3-months (93% vs 77%, p = 0.025); however, patient reported outcomes, complications and oncological outcomes were similar [701].
As there is conflicting evidence on the effect of anterior and/or posterior reconstruction on return to continence post-RP, no recommendations can be made. However, no studies showed an increase in adverse oncologic outcome or complications with reconstruction.
6.2.2.4.2. Deep venous thrombosis prophylaxis
As with all pelvic cancer surgery lasting over one hour there is a measurable increased risk of deep vein thrombosis and so consideration should be given to chemical thrombosis prophylaxis, commonly used for 3 to 4 weeks after surgery. This should be adapted based on national recommendations, when available.
6.2.3. Radiotherapy
Intensity-modulated RT (IMRT) or volumetric modulated arc therapy (VMAT) with image-guided RT (IGRT) is currently widely recognised as the standard treatment approach for EBRT.
6.2.3.1. External beam radiation therapy
6.2.3.1.1. Technical aspects
Intensity-modulated RT and VMAT employ dynamic multi-leaf collimators, which automatically and continuously adapt to the contours of the target volume seen by each beam. Viani et al., show significantly reduced acute and late grade ≥ 2 genito-urinary (GU) and gastro-intestinal (GI) toxicity in favour of IMRT, while BCR-free rates did not differ significantly when comparing IMRT with three-dimensional conformal RT (3D-CRT) in a RCT comprising 215 patients [702]. A meta-analysis by Yu et al., (23 studies, 9,556 patients) concluded that IMRT significantly decreases the occurrence of grade 2–4 acute GI toxicity, late GI toxicity and late rectal bleeding, and achieves better PSA relapse-free survival in comparison with 3D-CRT. Intensity-modulated EBRT and 3D-CRT show comparable acute rectal toxicity, late GU toxicity and OS, while IMRT slightly increases the morbidity of acute GU toxicity [703]. Thus, IMRT plus IGRT remain the SOC for the treatment of PCa.
The advantage of VMAT over IMRT is shorter treatment times, generally two to three minutes in total. Both techniques allow for a more complex distribution of the dose to be delivered and provide concave isodose curves, which are particularly useful as a means of sparing the rectum. Radiotherapy treatment planning for IMRT and VMAT differs from that used in conventional EBRT, requiring a computer system capable of ‘inverse planning’ and the appropriate physics expertise. Treatment plans must conform to pre-specified dose constraints to critical organs at risk of normal tissue damage and a formal quality assurance process should be routine.
With dose escalation using IMRT/VMAT, organ movement becomes a critical issue in terms of both tumour control and treatment toxicity. Techniques will therefore combine IMRT/VMAT with some form of IGRT (usually gold marker or cone-beam CT), in which organ movement can be visualised and corrected for in real time, although the optimum means (number of applications per week) of achieving this is still unclear [704,705]. Tomotherapy is another technique for the delivery of IMRT, using a linear accelerator mounted on a ring gantry that rotates as the patient is delivered through the centre of the ring, analogous to spiral CT scanning.
The use of MR-guided adapted RT is still investigational [706]. Planning studies confirm that MR-based adaptive RT significantly reduces doses to organs at risk (OAR) and this may translate into clinical benefit [707]. Although the rates of acute GI- and GU toxicity appear low, mostly on the basis of patients treated with stereotactic RT [708], follow-up is too short for definitive conclusions [706]. The daily fraction time of up to 45 minutes [706,708], the heavy MR-workflow and the limited field size (rendering most pelvic fields too large) make its implementation not yet a routine [706]. A prospective single center RCT, the MIRAGE trial (CT-guided Stereotactic Body Radiation Therapy and MRI-guided Stereotactic Body Radiation Therapy for Prostate Cancer) demonstrates reduced acute GU and GI toxicity with MRI-guided SBRT and margin reduction from 4 mm to 2 mm [709]. The impact on long term toxicity, biochemical control and cost effectiveness remains undefined.
6.2.3.1.2. Dose escalation
Local control is a critical issue for the outcome of RT of PCa. It has been shown that local failure due to insufficient total dose is prognostic for death from PCa as a second wave of metastases is seen five to ten years later on [710]. Several RCTs have shown that dose escalation (range 74–80 Gy) has a significant impact on ten-year biochemical relapse as well as metastases and disease-specific mortality [711-718]. These trials have generally included patients from several risk groups, and the use of neoadjuvant/adjuvant ADT has varied (see Table 6.2.6). The best evidence of an OS benefit in patients with intermediate- or high-risk PCa, derives from a non-randomised but well conducted propensity-matched retrospective analysis of the U.S. National Cancer Database by Kalbasi et al., including a total of 42,481 patients [719]. If IMRT/VMAT and IGRT are used for dose escalation, rates of severe late side effects (> grade 3) for the rectum are 2–4% and for the GU tract 2–6%
[713,720].
The concept of a focal boost to the dominant intraprostatic lesion (DIL) visible on MRI rather than global prostate dose escalation has been successfully validated in a RCT of 571 intermediate- and high-risk patients [720]. Patients were randomised between 77 Gy in 35 fractions of 2.2 Gy and the same dose plus a focal boost up to 18 Gy. Additional ADT was given to 65% of patients in both arms. However, the duration of the ADT was not reported. With a median follow-up of 72 months there was a moderate improvement of biochemical PFS (BPFS) (primary endpoint). In addition, focal boosting decreased local failure (HR: 0.33) and increased the rate of regional + distant MFS (HR: 0.58) [721]. No significant difference for late GU- or GI toxicity grade ≥ 2 (23% and 12% vs. 28% and 13%) was documented. For grade ≥ 3 GU-toxicity these numbers were 3.5% and 5.6% (p > 0.05). However, longer follow-up is needed to assess late GU-toxicity [721]. Of note, there was a clear decrease in biochemical failure with increasing boost dose, individually given up to 18 Gy. Systematic review of MRI-defined DIL focal boost studies using standard fractionation shows good tolerability and improved BPFS [722]. Its role when using hypofractionation and ultra-hypofractionation is under investigation.
6.2.3.1.3. Hypofractionation
Fractionated RT utilises differences in the DNA repair capacity of normal and tumour tissue and slowly proliferating cells are very sensitive to an increased dose per fraction [723]. A meta-analysis of 25 studies including > 14,000 patients concluded that since PCa has a slow proliferation rate, hypofractionated RT could be more effective than conventional fractions of 1.8–2 Gy [724]. Hypofractionation (HFX) has the added advantage of being more convenient for the patient at lower cost.
Moderate HFX is defined as RT with 2.5–3.4 Gy/fx. Several studies report on moderate HFX applied in various techniques also including ADT in part [725-732]. A Cochrane review on moderate HFX for clinically localised PCa [733] included eleven studies (n = 8,278) with a median follow-up of 72 months showing little or no difference in PCa-specific survival (HR: 1.00). Based on four studies (n = 3,848), moderate HFX to the protstate alone probably makes little or no difference to late radiation GU toxicity (RR: 1.05) or GI toxicity (RR: 1.1). Toxicity outcomes in two RCTs recruiting high risk patients and adding elective pelvic nodal radiation have reported. The PCS-5 multicentre RCT recruited high risk patients (25.9% T3-4) and an initial two-year toxicity analysis demonstrated comparable G2+ GI toxicity across treatment arms with lower rates of late G2+ GU toxicity with HFX [734]. No differences were seen in survival outcomes at median follow-up of five years, although as secondary endpoints extrapolation of survival results is limited by small sample size [735]. In the single centre randomized pHART2-RCT an increase in five-year G3+ GI toxicity was noted when HFX was combined with elective pelvic nodal RT [736]. In the post-operative setting, moderate HFX is non-inferior in terms of two-year patient reported toxicity to conventional fractionation with similar rates of patient reported GI and GU toxicity [737].
Ultra-HFX has been defined as RT with > 3.4 Gy per fraction [732]. It requires IGRT and (ideally) stereotactic body RT (SBRT). Table 6.2.8 provides an overview of selected studies investigating its role in treating predominantly intermediate risk localised disease. Short-term biochemical control (5-years) is comparable to conventional fractionation. However, there are concerns about higher-grade GU toxicity and SBRT should be avoided in patients with severe pre-existing LUTS and/or outflow obstruction with or without median lobe [738,739]. In the HYPO-RT-PC randomised trial by Widmark et al., (n = 1,200), no difference in failure-free survival was seen for conventional or ultra-HFX but acute grade ≥ 2 GU toxicity was 23% vs. 28% (p = 0.057), favouring conventional fractionation. There were no significant differences in long-term toxicity [738]. A SR by Jackson et al., included 38 studies with 6,116 patients who received RT with < 10 fractions and ≥ 5 Gy per fraction. Five and seven-year biochemical recurrence-free survival (BRFS) rates were 95.3% and 93.7%, respectively, and estimated late grade ≥ 3 GU and GI toxicity rates were 2.0% and 1.1%, respectively [740]. The authors conclude that there is sufficient evidence to support SBRT as a standard treatment option for localised PCa, even though the median follow-up in this review was only 39 months and it included at least one trial (HYPO-RT-PC) which used 3D-CRT in 80% and IMRT/VMAT in the remainder for ultra-HFX. In their review on SBRT, Cushman et al., evaluated fourteen trials, including 2,038 patients and concluded that despite a lack of long-term follow-up and the heterogeneity of the available evidence, prostate SBRT affords appropriate biochemical control with few high-grade toxicities [741]. In the Intensity-modulated fractionated RT vs. stereotactic body RT for PCa (PACE-B) trial, acute grade ≥ 2 GU or GI toxicities did not differ significantly between conventional fractionation and ultra- HFX [742]. At two years, treatment was well tolerated in both arms with no differences in RTOG ≥ Grade 2 GU or GI toxicities, but clinician scoring of urinary toxicity using CTCAE and patient reported Expanded Prostate Cancer Index Composite (EPIC)-26 urinary bother scores were both higher in the SBRT arm [743]. After 74 months median follow-up, 5-year biochemical/clinical failure free-rates were 94.6% (95% CI 91.9%, 96.4%) in the control arm and 95.8% (95% CI 93.3%, 97.4%) in the SBRT arm confirming SBRT is non-inferior (HR 0.73 90% CI 0.48-1.12, p for non-inferiority =0.004). The cumulative 5-year rate of late RTOG grade 2+ GI toxicity was similar in both arms (10%) but higher rates of cumulative 5-year RTOG grade2+ GU toxicity occurred with SBRT, at 26.9% (95%CI 22.8,31.5%) compared to the control arm at 18.3% (95%CI 14.8,22.5%). The GU toxicity is temporary with no statististical difference in clinician reported toxicity between groups at 5 years and no clinically relevant difference in patient reported outcomes in the five years of follow-up. Adopting planning dose constraints to the penile bulb might minimise ED, especially in younger patients [744].
First results of a small (n = 30) randomised phase-II trial in intermediate-risk PCa of ‘ultra-high single dose RT’ (SDRT) with 24 Gy compared with an ultra HFX stereotactic body RT regime with 5x9 Gy, have been published [745].
6.2.3.1.4 Neoadjuvant or adjuvant hormone therapy plus radiotherapy
The combination of RT with luteinising hormone releasing hormone (LHRH) ADT has superiority compared with RT alone followed by deferred ADT on relapse, as shown by phase III RCTs [745-756] (Table 6.2.9). The main message is that for intermediate-risk disease a short duration of four to six months is optimal while a longer one, two to three years, is needed for high-risk patients. The largest RCT in intermediate risk disease comparing dose escalated RT with or without six months of ADT failed to demonstrate an OS advantage with a median follow-up time of 6.3 years. Six months of ADT use was associated with reduced PSA failure, fewer distant metastases and improved prostate cancer specific mortality [756].
The question of the added value of EBRT combined with ADT has been clarified by three RCTs. All showed a clear benefit of adding EBRT to long-term ADT (Table 6.2.10).
The combination of ADT with various forms of RT has been extensively studied, with extremely strong evidence for the use of such combined modality therapy in several settings. The MARCAP (Individual Patient Data Meta- Analysis of Randomised Trials in Cancer of the Prostate) consortium conducted a meta-analysis of trials using individual patient data (IPD), and a primary endpoint of MFS, a validated surrogate for OS. Trials were eligible if they studied the use or prolongation of ADT in patients receiving definitive RT, and included twelve trials with 10,853 patients. Median follow-up was over eleven years. The use of ADT was clearly associated with significant improvements in BCR, metastatic recurrence, MFS, and OS. The benefits of ADT were independent of RT dose, age, and risk groups comparing NCCN unfavourable intermediate-risk, high-risk and locally-advanced disease. There were no demonstrable benefits from the extension of duration of neoadjuvant ADT [757].
A meta-analysis from two RCTs (RTOG 9413 and Ottawa 0101) has compared neoadjuvant/concomitant vs. adjuvant ADT (without substratifying between favourable- and unfavourable intermediaterisk disease) in conjunction with prostate RT and reported superior PFS with adjuvant ADT, but the data heterogeneity means that this observation is hypothesis-generating only [758].
In addition, a Canadian two-arm dose-escalated (76 Gy) RCT compared neoadjuvant and concomitant with adjuvant short-term ADT in 432 patients with intermediate-risk PCa. After ten years no significant difference in OS or RT-related grade ≥ 3 GI or GU toxicity was seen [759]. Therefore, both regimen in combination with dose escalation are reasonable standards.
6.2.3.2. Proton beam therapy
In theory, proton beams are an attractive alternative to photon-beam RT for PCa, as they deposit almost all their radiation dose at the end of the particle’s path in tissue (the Bragg peak), in contrast to photons which deposit radiation along their path. There is also a very sharp fall-off for proton beams beyond their deposition depth, meaning that critical normal tissues beyond this depth could be effectively spared. In contrast, photon beams continue to deposit energy until they leave the body, including an exit dose. The PARTIQoL Phase III RCT compared proton beam therapy (PBT) with IMRT in 450 participants with localised prostate cancer. With a median follow-up of 60.3 months, no difference in any QoL domain or PFS was found [760]. Proton beam therapy has no advantages over less resource intensive IMRT/VMAT; however, the publication of the full study is awaited to confirm the results.
Table 6.2.6: Randomised trials of dose escalation in localised PCa
| Randomised trials of dose escalation in localised PCa | ||||||
| Trial | n | PCa condition | Radiotherapy Dose | Follow-up (median) | Outcome | Results |
MD Anderson study 2011 [718] | 301 | T1-T3, N0, M0, PSA ≤ 10 ng/mL PSA 10-20 ng/mL PSA > 20 ng/mL | 70 vs.78 Gy | 15 yr. | DM, DSM, FFF | All patients: 18.9% FFF at 70 Gy; 12% FFF at 78 Gy; (p = 0.042) 3.4% DM at 70 Gy; 1.1% DM at 78 Gy; (p = 0.018) 6.2% DSM at 70 Gy; 3.2% DSM at 78 Gy; (p = 0.043) No difference in OS (p > 0.05) |
PROG 95-09 2010 [712] | 393 | T1b-T2b, PSA ≤ 15 ng/mL 75% low-risk pts. Low-risk: T1-2a, PSA < 10 mg/mL, GS ≤ 6. Interm-risk: PSA 10-15 ng/mL or GS 7 or T2b. High-risk: GS 8-10. | 70.2 vs.79.2 Gy including proton boost 19.8 vs. 28.8 Gy | 8.9 yr. | 10-yr. ASTRO BCF | All patients: 32% BF at 70.2 Gy; 17% BF at 79.2 Gy; (p < 0.0001) Low-risk patients: 28% BF at 70.2 Gy; 7% BF at 79.2 Gy; (p < 0.0001) |
| MRC RT01 2014 [717] | 843 | T1b-T3a, N0, M0 PSA < 50 ng/mL neoadjuvant ADT | 64 vs. 74 Gy | 10 yr. | BFS, OS | 43% BFS at 64 Gy; 55% BFS at 74 Gy; (p = 0.0003) 71% OS both groups (p = 0.96) |
| Dutch RCT 2014 [716] | 664 | T1b-T4 143 pts. with (neo) adjuvant ADT | 68 vs. 78 Gy | 110 mo. | Freedom biochemical (Phoenix) and/or clinical failure at 10 yr. | 43% FFF at 68 Gy; 49% FFF at 78 Gy; (p = 0.045) |
GETUG 06 2011 [715] | 306 | T1b-T3a, N0, M0 PSA < 50 ng/mL | 70 vs. 80 Gy | 61 mo. | BCF (ASTRO) | 39% BF at 70 Gy; 28% BF at 80 Gy |
RTOG 0126 2018 [711] | 1,532 | T1b-T2b ISUP GG 1 + PSA 10-20 ng/mL or ISUP GG 2/3 + PSA < 15 ng/mL | 70.2 vs. 79.2 Gy | 100 mo. | OS, DM, BCF (ASTRO) | 75% OS at 70.2 Gy; 76% OS at 79.2 Gy 6% DM at 70.2 Gy; 4% DM at 79.2 Gy; (p = 0.05) 47% BCF at 70.2 Gy; 31% BCF at 79.2 Gy; (p < 0.001; Phoenix, p < 0.001) |
| FLAME Trial [720,721] | 571 | EAU risk classification: Intermediate risk (15%) High risk (84%) | 77 Gy (35 Fx. 2.2 Gy) vs. 77 Gy 35 Fx.) + focal boost (up to 18 Gy) ADT (65% both arms - duration unknown) | 72 mo. | BFS (5 yr.) DSM (5 yr.) | BFS: 92% at 77 Gy + boost; 85% at 77 Gy; (p < 0.001, HR: 0.45) DSM: p= 0.49 Focal boost in favour of: Local control (HR: 0.33); Distant MFS (HR: 0.58) |
ADT = androgen-deprivation therapy; BF = biochemical failure; BFS = biochemical progression-free survival; DM = distant metastases; DSM = 50disease specific mortality; FFF = freedom from biochemical or clinical failure; Fx = fractions; GS = Gleason score; ISUP = International Society of Urological Pathology; MFS = metastasis-free survival; mo. = months; n = number of patients; OS = overall survival; PSA = prostate-specific antigen; yr. = year.
Table 6.2.7: Major phase III randomised trials of moderate hypofractionation for primary treatment
| Study/Author | n | Risk, ISUP GG, or NCCN | ADT | RT Regimen | BED, Gy | Follow-up (median) | Outcome |
Lee, et al. 2024 [761] | 550 542 | low risk | None | 70 Gy/28 fx 73.8 Gy/41 fx | 80 69.6 | 150 mo. | 12 yr. DFS 56.1% (95% CI, 51.5 to 60.5) control arm and 61.8% (95% CI, 57.2 to 66.0) for HFX. HR 0.85 (95% CI, 0.71 to 1.03) |
Dearnaley, et al. CHHiP 2016 [728] | 1,077/19 fx 1,074/20 fx 1,065/37 fx | 15% low 73% intermediate 12% high | 3-6 mo. before and during EBRT | 57 Gy/19 fx 60 Gy/20 fx 74 Gy/37 fx | 73.3 77.1 74 | 62 mo. | 5 yr. BCDF 85.9% (19 fx) 90.6% (20 fx) 88.3% (37 fx) |
| De Vries, et al. 2020 | 403 392 | 30% ISUP GG 1 45% ISUP GG 2-3, 25% ISUP GG 4-5 | None | 64.6 Gy/19 fx 78 Gy/39 fx | 90.4 78 | 89 mo. | 8-yr. OS 80.8% vs. 77.6% (p = 0.17) 8 yr. TF 24.4% vs. 26.3% |
Catton, et al. 2017 [730] | 608 | Intermediate risk 53% T1c 46% T2a-c | None | 60 Gy/20 fx | 77.1 | 72 mo. | 5 yr. BCDF both arms 85% HR: 0.96 (n.s)
|
| 598 | 9% ISUP GG 1 63% ISUP GG 2 28% ISUP GG 3 | 77.1 78 Gy/39 fx | 78 | ||||
Glicksman PHART-2 [736] | 186 | All high risk N0M0 T1-2 82.8% T3-4 12.2% | 22 mo. median | 68Gy to prostate (SIB) + 48Gy to pelvis in 25 fx
78Gy to prostate + 46Gy to pelvis in 39 fx | 82
78 | 67 mo. | No difference in acute toxicity and PROs Higher 5-yr cumulative G3+ GI in HFX 13.5% (95% CI, 7.1%-21.9%) vs 2.4% (95% CI, 0.5%-7.6%) (P = .01) |
Niazi, et al. 2023 PCS-5 [734] | 329 | All high risk N0M0 T1-2 73.8% T3-4 25.9% | 28 mo. - 3 mo. before during and after EBRT | 68Gy to prostate (SIB) + 45Gy to pelvis in 25 fx
76Gy to prostate + 46Gy to pelvis in 38 fx | 82
76 | 24 mo. | Similar 2yr G2+ GI toxicity (8-10%)
Reduced 2yr G2+ GU toxicity with HFX (4.3% vs 15.9%; p=0.035) |
ADT = androgen deprivation therapy; BCDF = biochemical or clinical disease failure; BED = biologically equivalent dose, calculated to be equivalent in 2 Gy fractions using an α/ß of 1.5 Gy; DFS = diseasefree survival; EBRT = external beam radiotherapy; HFX = hypofractionation; FU = follow-up; fx = fractions; HR = hazard ratio; ISUP = International Society of Urological Pathology; mo. = month; n = number of patients; NCCN = National Comprehensive Cancer Network; n.s. = not significant; TF = treatment failure; yr. = year.
Table 6.2.8: Selected trials on ultra-hypofractionation for intact localised PCa
| Study | n | med FU (mo) | Risk-Group | Regimen (TD/fx) | Outcome |
| Widmark et al. 2019 HYPO-RT-PC [738] | 1,200 | 60 | 89% intermediate 11% high | 78 Gy / 39 fx, 8 wks 42.7 Gy / 7 fx, 2.5 wks No SBRT | FFS at 5 yrs 84% in both arms |
Brand et al. 2019 Tree et al. 2022 Van As et al. 2024 [743] | 874 | 74 | 9.3% NCCN low 90.7% NCCN intermediate ISUP GG 3 excluded | 78 Gy / 39 fx, 7.5 wks or 62 Gy/ 20 fx 4wks 36.25 Gy / 5 fx, 1-2 wks SBRT | Biochemical/clinical FFS at 5 yrs 94.6% (CRT) vs. 95.6% (SBRT) Cumulative 5-yr G 2+ GI toxicity similar (10%) Cumulative 5-yr G2+ GU SBRT 26.9% (95%CI 22.8,31.5%) CRT 18.3% (95%CI 14.8,22.5%). |
FFS = failure-free survival; FU = follow-up; fx = number fractions; mo. = months; n = number of patients; TD = total dose; SBRT = stereotactic body radiotherapy; CRT = control arm RT; wk. = weeks; yr. = years; ns=not significant.
Table 6.2.9: Selected studies of use and duration of ADT in combination with RT for PCa
| Selected studies of use and duration of ADT in combination with RT for PCa | ||||||
| Study | TNM stage | n | Trial | ADT | RT | Effect on OS |
RTOG 85-31 2005 [747] | T3 or N1 M0 | 977 | EBRT ± ADT | Orchiectomy or LHRH agonist 15% RP | 65–70 Gy | Significant benefit for combined treatment (p = 0.002) seems to be mostly caused by patients with ISUP grade group 2-5 |
RTOG 94-13 2007 [751] | T1c–4 N0–1 M0 | 1,292 | ADT timing comparison | 2 mo. neoadjuvant plus concomitant vs. 4 mo. Adjuvant suppression | Whole pelvic RT vs. prostate only; 70.2 Gy | No significant difference between neoadjuvant plus concomitant vs. adjuvant androgen suppression therapy groups (interaction suspected) |
RTOG 86-10 2008 [748] | T2–4 N0–1 | 456 | EBRT ± ADT | Goserelin plus flutamide 2 mo. before, plus Concomitant therapy | 65–70 Gy RT | No significant difference at 10 yr. |
D’Amico AV, et al. 2008 [749] | T2 N0 M0 (localised unfavourable risk) | 206 | EBRT ± ADT | LHRH agonist plus flutamide for 6 mo. | 70 Gy 3D-CRT | Significant benefit that may pertain only to men with no or minimal co-morbidity (HR: 0.55, 95% CI: 0.34-0.90, p = 0.01) |
RTOG 92-02 2008 [752] | T2c–4 N0–1 M0 | 1,554 | Short vs. prolonged ADT | LHRH agonist given for 2 yr. as adjuvant after 4 mo. as neoadjuvant | 65–70 Gy | p = 0.73, p = 0.36 overall; significant benefit (p = 0.044) (p = 0.0061) in subset with ISUP grade group 4-5 |
| EORTC 22961 2009 [753] | T1c-2ab N1 M0, T2c-4 N0-1 M0 | 970 | Short vs. prolonged ADT | LHRH agonist for 6 mo. vs. 3 yr. | 70 Gy 3D-CRT | Better result with 3 yr. treatment than with 6 mo. (3.8% improvement in survival at 5 yr.) |
EORTC 22863 2010 [746] | T1-2 poorly differentiated and M0, or T3-4 N0-1 M0 | 415 | EBRT ± ADT | LHRH agonist for 3 yr. (adjuvant) | 70 Gy RT | Significant benefit at 10 yr. for combined treatment (HR: 0.60, 95% CI: 0.45-0.80, p = 0.0004). |
| TROG 96-01 2011 [750] | T2b–4 N0 M0 | 802 | Neoadjuvant ADT Duration | Goserelin plus flutamide 3 or 6 mo. before, plus concomitant suppression | 66 Gy 3D-CRT | No significant difference in OS reported; benefit in PCa-specific survival (HR: 0.56, 95% CI: 0.32-0.98, p = 0.04) (10 yr.: HR: 0.84, 0.65-1.08, p = 0.18) |
RTOG 99-10 2015 [754] | intermediate risk 94% T1-T2; 6% T3-4 | 1,579 | Short vs. prolonged ADT | LHRH + bicalutamide 6 mo. 4 mo.prior to RT | 70.2 Gy 2D/3D | 67 vs. 68%, p = 0.62, confirms 8 + 8 wk. LHRH as a standard |
| PCSIII 2020 [755] | Intermediate risk | 600 | 76 Gy alone vs. 76 Gy + ADT vs. 70 Gy + ADT | LHRH + bicalutamide 6 mo. 4 mo. prior to RT | 70 vs. 76 Gy | Significantly improved biochemical failure-free and PCa-specific survival for ADT arms, with no difference in OS. |
RTOG 0815 2023 [756] | Intermediate risk | 1,492 | Dose escalated RT ± ADT | LHRH agonist/antagonist + bicalutamide or flutamide 6 mo. 2 mo. prior to RT | 79.2Gy (89%) 45Gy + BT boost (11%) | No difference in OS. Significantly improved biochemical failure-free, metastatic-free survival and PCa-specific survival for ADT arm. |
ADT = androgen deprivation therapy; CI = confidence interval; EBRT = external beam radiotherapy in standard fractionation; HR = hazard ratio; ISUP = International Society of Urological Patholohy; LHRH = luteinising hormone-releasing hormone; mo. = months; n = number of patients; OS = overall survival; RP = radical prostatectomy; RT = radiotherapy; BT = brachytherapy; wk = week; yr. = year; 3D-CRT = three-dimensional conformal radiotherapy.
Table 6.2.10: Selected studies of ADT in combination with, or without, RT for PCa
| Selected studies of ADT in combination with, or without, RT for PCa | ||||||
| Study | TNM stage | n | Trial design | ADT | RT | Effect on OS |
| SPCG-7/SFUO-3 2016 [763] | T1b-2 WHO Grade 1-3, T3 N0 M0 | 875 | ADT ± EBRT | LHRH agonist for 3 mo. Plus continuous flutamide | 70 Gy 3D-CRT vs. no RT | 34% (95% CI: 29-39%) vs. 17% (95% CI: 13-22% CSM at 12 (15) yr. favouring combined treatment (p < 0.0001 for 15-yr. results) NCIC CTG PR.3/MRC |
| PRO7/NCIC 2015 [764] | T3-4 (88%), PSA > 20 ng/mL (64%), ISUP GG 4-5 (36%) N0 M0 | 1,205 | ADT ± EBRT | Continuous LHRH agonist | 65–70 Gy 3D-CRT vs. no RT | 10-yr. OS = 49% vs. 55% favouring combined treatment HR: 0.7, p < 0.001) |
| Sargos, et al., 2020 [765] | T3-4 N0 M0 | 273 | ADT ± EBRT | LHRH agonist for 3 yr. | 70 Gy 3D-CRT vs. no RT | Significant reduction of clinical progression; 5-yr. OS 71.4% vs. 71.5% |
ADT = androgen-deprivation therapy; CSM = cancer-specific mortality; EBRT = external beam radiotherapy; HR = hazard ratio; LHRH = luteinising hormone-releasing hormone; mo. = months; n = number of patients; OS = overall survival; PSA = prostate-specific antigen; RT = radiotherapy; 3D-CRT = three-dimensional conformal radiotherapy;yr = years.
6.2.3.3. Spacer during external beam radiation therapy
Biodegradable spacer insertion involves using a liquid gel or balloon to increase the distance between the prostate and rectum and consequently reduce the amount of radiation reaching the rectum. Various materials have been used with most evidence available for CE-marked hydrogel spacers [766]. A meta-analysis including one RCT and six cohort studies using the hydrogel spacer demonstrated a 5–8% reduction in the rectal volume receiving high-dose radiation, although heterogeneity between studies is found [767]. In the final analysis of the RCT with a median follow-up of 37 months and with approximately two-thirds of patients evaluable, those treated with spacer in situ had no deterioration from baseline bowel function whilst those treated without spacer had a lower mean bowel summary score of 5.8 points which met the threshold for a minimally important difference of 4–6 points [768].
This meta-analysis highlights inconsistent reporting of procedural complications. In addition, with more widespread clinical use safety reports describe uncommon, but severe and life changing, complications including prostatic abscess, fistulae and sepsis [769]. Implantation is associated with a learning curve and should only be undertaken by teams with experience of TRUS and transperineal procedures with robust audit reporting in place [769]. Its role in the context of moderate or extreme HFX is as yet unclear.
6.2.3.4. Brachytherapy
6.2.3.4.1. Low-dose rate brachytherapy
Low-dose rate (LDR) BT uses radioactive seeds permanently implanted into the prostate. Low-dose rate monotherapy [770] can be offered to patients with NCCN favourable intermediate-risk and good urinary function defined as an International Prostatic Symptom Score (IPSS) < 12 and maximum flow rate > 15 mL/min on urinary flow tests [771]. The RTOG phase III RCT compared LDR BT +/- EBRT in participants with Gleason grade 6 and PSA < 20 or Gleason grade 7 and PSA < 10 and found that the addition of EBRT resulted in increased toxicity but no improvement in freedom from progression [772].
Patients having had a previous TURP can undergo BT without an increase in risk of urinary toxicity with due attention to dose distribution. A minimal channel TURP is recommended, leaving at least 1 cm rim of prostate tissue around the post-TURP urethral defect at the postero-lateral sides of the prostate and there should be at least a three-month interval between TURP and BT to allow for adequate healing [773-776].
The only available RCT comparing RP and LDR BT as monotherapy was closed due to poor accrual [777]. Outcome data are available from a number of large population cohorts with mature follow-up [778-782]. A significant correlation has been shown between the implanted dose and biochemical control [783]. A D90 (dose covering 90% of the prostate volume) of > 140 Gy leads to a significantly higher biochemical control rate (PSA < 1.0 ng/mL) after four years (92 vs. 68%). There is no OS benefit in adding neoadjuvant or adjuvant ADT to LDR monotherapy [784].
Low-dose rate BT can be combined with EBRT in NCCN unfavourable intermediate-risk PCa and high-risk patients. External beam RT (total dose of 78 Gy) has been compared with EBRT (total dose 46 Gy) followed by LDR BT boost (prescribed dose 115 Gy) in intermediate-risk and high-risk patients in the ASCENDE-RT randomised trial with twelve months of ADT in both arms [785,786]. The LDR boost resulted in 5-, 7-year and 10-year PSA PFS increase (89%, 86% and 85% respectively, compared to 84%, 75%, 70%) but with no impact on distant metastasis or OS. This improvement in biochemical control was achieved at a cost of increased late grade 3+ GU toxicity (18% compared to 8%) and two treatment related deaths [786,787]. Urinary toxicity was mainly in the development of urethral strictures and incontinence and great care should be taken during treatment planning.
6.2.3.4.2. High-dose rate brachytherapy
High-dose rate (HDR) BT uses a radioactive source temporarily introduced into the prostate to deliver radiation. The technical differences are outlined in Table 6.2.11. The use of the GEC (Groupe Europeen de Curietherapie)/ESTRO Guidelines is strongly recommended [788]. High-dose rate BT can be delivered in single or multiple fractions and is often combined with EBRT of at least 45 Gy, conventionally fractionated [789]. A retrospective analysis on 1641 intermediate and high-risk patients demonstrated a better distant-metastasis free survival when a HDR BT boost was added to 50 – 54 Gy EBRT. The difference mounted to 12% at ten years [790]. A SR of non-RCTs and data from population studies suggest outcomes with EBRT plus HDR BT are superior to EBRT alone [791,792].
A single-centre RCT of EBRT (55 Gy in 20 fractions) vs. EBRT (35.75 Gy in 13 fractions), followed by HDR BT (17 Gy in two fractions over 24 hours) has been reported [793]. In 218 patients with T1–3 N0M0 PCa the combination of EBRT and HDR BT showed a significant improvement in the biochemical disease-free rate (p = 0.04) at five and ten years (75% and 46% compared to 61% and 39%). However, an unexpectedly high rate of early recurrences was observed in the EBRT arm alone, even after two years, possibly due to a dose lower than the current standard used [793].
Supporting, but not definitive, evidence of the benefit of HDR boost is available from the TROG 03.04 RADAR trial. This multi-centre study had upfront radiation dose escalation (non-randomised) with dosing options of 66, 70, or 74 Gy EBRT, or 46 Gy EBRT plus HDR BT boost and randomised men with locally-advanced PCa to 6 or 18 months ADT. After a minimum follow-up of ten years HDR boost significantly reduced distant progression, the study primary endpoint (HR: 0.68, 95% CI: 0.57–0.80; p < 0.0001), when compared to EBRT alone and, independent of duration of ADT, HDR boost was associated with increased IPSS of 3 points at eighteen months post-treatment resolving by three years but decreased rectal symptoms when compared to EBRT [794]. Although radiation dose escalation using BT boost provides much higher biological doses, the TROG 03.04 RADAR RCT and SRs show ADT use independently predicts better outcomes regardless of radiation dose intensification [784,794,795]. Omitting ADT may result in inferior OS and based on current evidence ADT use and duration should be in line with that used when delivering EBRT alone.
Fractionated HDR BT as monotherapy can be offered to patients with intermediate-risk PCa, who should be informed that results are only available from limited series in very experienced centres. Five-year PSA control rates of 93.5% for intermediate-risk PCa are reported, with late grade 3+ GU toxicity rates < 5% and no, or very minimal, grade 3+ GI toxicity rates [796]. Single fraction HDR monotherapy should not be used as it has inferior biochemical control rates compared to fractionated HDR monotherapy [797].
Table 6.2.11: Difference between LDR and HDR brachytherapy
| Differences in prostate brachytherapy techniques | |
| Low dose rate (LDR) |
|
| High dose rate (HDR) |
|
6.2.3.5. Acute side effects of external beam radiotherapy and brachytherapy
Gastro-intestinal and urinary side effects are common during and after EBRT. In the EORTC 22991 trial, approximately 50% of patients reported acute GU toxicity of grade 1, 20% of grade 2, and 2% grade 3. In the same trial, approximately 30% of patients reported acute grade 1 GI toxicity, 10% grade 2, and less than 1% grade 3. Common toxicities included dysuria, urinary frequency, urinary retention, haematuria, diarrhoea, rectal bleeding and proctitis [798]. In addition, general side effects such as fatigue are common. It should be noted that the incidence of acute side effects is greater than that of late effects, implying that most acute effects resolve.
In a RCT comparing patient reported QoL after LDR or HDR boost combined with external beam radiotherapy to the pelvis, more intense and prolonged acute urinary side-effects are noted with LDR boost [799]. In a RCT of conventional dose EBRT vs. EBRT and LDR BT the incidence of acute proctitis was reduced in the BT arm, but other acute toxicities were equivalent [785]. In a pooled analysis of 864 patients treated using extreme HFX and stereotactic RT, declines in urinary and bowel domains were noted at three months which returned to baseline, or better, by six months [800].
6.2.4. Investigational therapies
6.2.4.1. Background
Besides RP, EBRT and BT, other modalities have emerged as potential therapeutic options in patients with clinically localised PCa [801-803]. These new modalities have been developed as minimally invasive procedures with the aim of providing equivalent oncological safety, reduced toxicity, and improved functional outcomes. In this section, both whole gland- and focal treatment [804,805] will be considered, looking particularly at high-intensity focused US (HIFU), cryotherapeutic ablation of the prostate (cryotherapy) and focal photodynamic therapy (PDT), as sufficient data are available to form the basis of some initial judgements. Other options such as radiofrequency ablation (RFA) and electroporation, among others, are considered to be in the early phases of evaluation [804].
High-intensity focused US consists of focused US waves emitted from a transducer that cause tissue damage by mechanical and thermal effects as well as by cavitation [806]. The goal of HIFU is to heat malignant tissue above 65°C, so that it is destroyed by coagulative necrosis. High-intensity focused US is performed under general or spinal anaesthesia, with the patient lying in the lateral or supine position. Since the ultrasound energy is most often delivered from the rectal cavity, HIFU faces challenges in delivering energy to the anterior part in large prostates.
Cryotherapy uses freezing techniques to induce cell death by dehydration resulting in protein denaturation, direct rupture of cellular membranes by ice crystals and vascular stasis and microthrombi, resulting in stagnation of the microcirculation with consecutive ischaemic apoptosis [801-803]. Freezing of the prostate is ensured by the placement of 17-gauge cryo-needles under TRUS guidance, placement of thermosensors at the level of the external sphincter and rectal wall, and insertion of a urethral warmer. Two freeze-thaw cycles are used under TRUS guidance resulting in a temperature of -40°C in the mid-gland and at the neurovascular bundle. Currently, third and fourth generation cryotherapy devices are mainly used.
6.2.4.2. Whole-gland therapies
Whole gland treatments using cryosurgery and HIFU were investigated as a replacement for surgery or radiotherapy, with limited success. The main adverse effects of whole-gland cryosurgery are ED (18%), urinary incontinence (2–20%), urethral sloughing (0–38%), rectal pain and bleeding (3%) and recto-urethral fistula formation (0–6%) [807]. There is a lack of prospective comparative data regarding oncological outcomes of whole-gland cryosurgery as a curative treatment option for men with localised PCa, with most studies being non-comparative single-arm case series with short follow-up [807].
High-intensity focused US has previously been widely used for whole-gland therapy with the following adverse effects: acute urinary retention (10%), ED (23%), urethral stricture (8%), rectal pain or bleeding (11%), recto-urethral fistula (0–5%) and urinary incontinence (10%) [807]. Combining the whole-gland HIFU treatment with TURP reduces the rate of urethral strictures, maintains the level of incontinence, but increases the rate of ED [808].
Overall, the lack of any long-term prospective comparative studies, and data to suggest poor long-term oncological outcomes for men with high-risk localised disease [809] prevents whole-gland HIFU from being considered as a reasonable alternative to the established curative treatment options [807]. In addition, the expected improvements in functional outcome failed to materialise with 12% of patient developing incontinence and 61% developing ED [810].
6.2.4.3. Focal therapy
During the past two decades, there has been a trend towards earlier diagnosis of PCa as a result of greater public and professional awareness leading to the adoption of both formal and informal screening strategies. The effect of this has been that men are identified at an earlier stage with smaller tumours, with a greater propensity for unifocal disease [811-813]. There is also greater awareness of the risks of the consequences of treatment leading to attempts to ablate only a region of the prostate containing the tumour thereby limiting toxicity by sparing the neurovascular bundles, sphincter, and urethra [814-816]. The question remains which if any of these small unifocal tumours need treatment.
A SR included data from 5,827 patients across 72 studies and covered different energy sources including HIFU, cryotherapy, Photodynamic Therapy (PDT), laser interstitial thermotherapy, focal BT, irreversible electroporation (IRE) and radiofrequency ablation (RFA) [817]. The review favours HIFU and PDT for their higher quality data, over 95% of pad-free incontinence and 85–90% of patients without clinically significant cancer in short-term analysis. This has to be critically analysed, because 45% of all patients with a focal approach included in this SR had an ISUP Grade GG 1 cancer. The overall quality of the evidence was low, due to the majority of studies being single-centre, non-comparative and retrospective in design, heterogeneity of definitions and approaches, follow-up strategies, outcomes, and duration of follow-up. Although the review finds high quality evidence that focal therapy has favourable functional outcomes and minimises AEs, definitive evidence of oncological benefit remains unavailable.
A more stringent SR including only prospective studies and per protocol post-treatment biopsies found that after 1 year 8.8% of patients had an infield failure with ≥ ISUP GG 2 cancers and 13.0% had ≥ ISUP GG 2 cancers anywhere in the prostate [818]. This work did not include any definition of clinical relevant cancer and included 35% of patients with ISUP GG 1 at initial diagnosis. Focal ablation showed only 9% reduction in sexual function scores, compared to 43% for whole gland ablation, at one year.
At this time, the largest analysis on oncologic outcomes following focal HIFU includes 1,379 men with a median follow-up of 32 months (65% of patients were D’Amico intermediate risk and 28% high risk) [819]. In this study, one repeated focal HIFU session was allowed and performed in 18% of all patients. Parametric MRI was performed if consecutive PSA rises were identified and biopsies were offered if the mpMRI was suspicious. Eighty percent of patients had at least one follow-up mpMRI and 44% had a follow-up biopsy. The primary outcome was failure-free survival (FFS) which was defined as evidence of cancer requiring whole-gland salvage treatment. At 7 years the FFS for intermediate- and high-risk cancers was 68% and 65%, respectively [819].
At present, there is no well-defined pathway for focal therapy or follow-up and the field is still developing. The optimal energy source for tumours at different locations, the need for double treatments during initial therapy, the use of MRI or PSA for follow-up are still a matter of research. The guideline panel acknowledges the challenges for interventional RCTs [820-822]. The interim analysis and meeting reports demonstrate slow recruitment, patients declining consent and rejecting their treatment allocation into the RP group (approx. 25%). In an attempt to overcome this propensity-matched analysis using prospective multi-centre databases have been performed for comparison of focal therapy vs. radical therapy [823,824]. Such analyses are always susceptible to unmeasured selection biases in who was selected for each treatment.
Oncological follow-up data up to eight years can be used to counsel patients in treatment decisions [823,824]. Patients managed by focal therapy had a HIFU or cryotherapy, with one retreatment, if needed. Of these 17.1% of patients in the focal arm received a retreatment. The primary outcome was FFS defined as “need for local or systemic salvage treatment or metastasis”. Both groups included 246 patients with an average PSA of 7.9 ng/mL and 60% ISUP GG 2/3 cancers. The cancer core length was 5–6 mm with 45% having bilateral cancer. The authors report similar cancer control 8 years after therapy, with FFS and BCR of 83% and 23.9% for focal therapy vs. 79% and 24.8% for RP, respectively. Similar results were demonstrated in a cohort-based analysis with a follow-up six years [824]. The use of different definitions for oncological failure in the two arms is another limitation of these studies. While any recurrence after RP was seen as failure, a second HIFU was permitted in the focal group. The current data from the HIFU Evaluation and Assessment of Treatment (HEAT) registry indicates that a repeat-HIFU does not significantly decrease urinary or erectile function [825]. However, this change of failure definition will have to be re-evaluated. It is important to note, that these results were achieved in centres with a dedicated focal program where all patients had a mpMRI with targeted and systematic biopsies or full template mapping biopsies. Therefore, it seems necessary to perform systematic biopsies in patients, who are candidates for focal therapy.
The impact of salvage therapies after focal therapy was investigated in small series in specialized centres [826,827]. If a salvage RP is necessary after focal therapy, the reported functional and oncological outcomes are comparable to treatment-naive patients [828,829]. In a recent SR including 482 patients from twelve studies, the authors conclude that, when compared to primary surgery, the salvage radical prostatectomy after focal therapy has a higher PSM rate of 27% and a slightly worse incontinence rate. Although the early complication rate was also higher, most of them could be managed conservatively [830].
One comparative RCT was conducted in a very-low risk population, for which there is currently a strong movement away from any form of active treatment. This study was comparing padeliporfin-based vascular targeted PDT vs. AS and found at a median follow-up of 24 months that less patients progressed in the PDT arm compared with the AS arm (adjusted HR: 0.34, 95% CI: 0.24–0.46), and needed less radical therapy (6% vs. 29%, p < 0.0001). Updated results were published in 2018 showing that these benefits were maintained after four years [831]. Nevertheless, limitations of the study include an unusually high observed rate of disease progression in the AS arm (58% in two years) and more patients in the AS arm chose to undergo radical therapy without a clinical indication which may have introduced confounding bias. Finally, the AS arm did not undergo any confirmatory biopsy or any MRI scanning, which is not representative of contemporary practice. A matched-pair analysis comparing focal cryotherapy to AS with 76% ISUP GG 1 cancers failed to demonstrate any significant advantages for MFS and OS [832].
The available evidence indicates that focal therapy is associated with less AEs than whole gland or radical treatments. Many of the patients included in these trials would currently be considered to have been over treated. Robust prospective trials reporting standardised fifteen-year oncological outcomes [833] are needed in patients with clinically significant cancers before unrestricted recommendations in support of focal therapy for routine clinical practice can be made [804,833,834]. Currently, focal therapy using HIFU or cryotherapy should be performed within the context of a prospective registry.
All other ablative modalities and treatment strategies should only be offered in well-designed prospective trial setting. In order to allow quality analysis of the collected data, the prospective registry should adhere to the EMA recommendations (Guideline on registry-based studies EMA/426390/2021), which emphasises the need for clear follow-up timelines and timely recording, completeness of core data of consecutive patients enrolled, an analysis plan in defined intervals and a data quality management.
6.3. Treatment by disease stages
6.3.1. Management of low-risk disease
6.3.1.1. Watchful waiting
For patients with a life expectancy of < 10 years (based on co-morbidities and age), where curative treatment would not be an option in the case of progression after AS, WW is standard of care.
6.3.1.2. Active surveillance
Active surveillance should be considered standard of care for all patients with a life expectancy > 10 years (based on co-morbidities and age) and where curative treatment would be considered in the case of disease progression.
6.3.1.2.1. Androgen deprivation monotherapy
The Early Prostate Cancer (EPC) Trial Programme found that in patients with localised disease, ADT monotherapy did not improve PFS or OS in any of the subgroups, compared with placebo [835]. Instead, there was a statistically insignificant numerical trend towards worse OS with ADT in the WW sub-group (HR: 1.16, 95% CI: 0.99–1.37; p = 0.07). Although the trial did not directly address men with low-risk disease, it offered some evidence suggesting that otherwise asymptomatic men with localised disease should not receive ADT monotherapy.
6.3.1.3. Other therapeutic options
Other treatments such as whole-gland therapy (e.g. RP or RT) or focal ablative therapy remain highly likely to be overtreatment in the setting of low-risk disease and should not be used outside a trial setting.
6.3.1.4. Recommendations for the management of low-risk disease
| Recommendations | Strength rating |
| Manage patients with a life expectancy < 10 years by watchful waiting. | Strong |
| Manage patients with a life expectancy > 10 years and low-risk disease by active surveillance. | Strong |
6.3.2. Management of Intermediate-risk disease
6.3.2.1. Watchful waiting
For patients with a life expectancy of < 10 years (based on co-morbidities and age, where curative treatment is not a direct option or would not be an option in the case of progression after AS, WW is standard of care.
6.3.2.2. Active Surveillance
Although men with less favourable disease characteristics have worse outcomes after any treatment, the question is whether a delay in curative treatment due to initial AS, leads to additionally unfavourable outcomes. Intuitively, the higher risk disease, the higher risk of adverse outcomes due to an initial delay. Inclusion is based on favourable disease characteristics as discussed in section 6.2.1.2.2.
6.3.2.3. Radical prostatectomy
Patients with intermediate-risk PCa should be informed about the results of two RCTs (SPCG-4 and PIVOT) comparing RRP vs. WW in localised PCa. In the SPCG-4 study, death from any cause (RR: 0.71, 95% CI: 0.53–0.95), death from PCa (RR: 0.38, 95% CI: 0.23–0.62) and distant metastases (RR: 0.49, 95% CI: 0.32–0.74) were significantly reduced in intermediate-risk PCa at 18 years. After 30 years follow-up overall (not risk-stratified), RP reduced death from any cause (RR: 0.74, 95% CI: 0.64–0.87), death from PCa (RR: 0.52, 95% CI: 0.40–0.67) for a mean of 2.2 life-years (95% CI: 1.4-2.9) gained. In the PIVOT trial, according to a pre-planned subgroup analysis among men with intermediate-risk tumours, RP significantly reduced all-cause mortality (HR: 0.69, 95% CI: 0.49–0.98), but not death from PCa (0.50, 95% CI: 0.21–1.21) at ten years [836]. In the ProtecT trial, 24% of the population were intermediate risk (at baseline) and no significant difference in prostate cancer deaths was seen for RP versus active monitoring (with delayed active treatment, HR 0.68 (0.11–4.05). A meta-analysis based on the findings of SPCG-4, PIVOT and ProtecT demonstrated a benefit from RP over observation with a significantly decreased risk of death of 9% and of disease progression of 43% [837]. However, no stratification by disease stages was performed. A large study found 2.9% of LN invasion in a contemporary cohort of 6,883 patients undergoing RP and LND for intermediate risk PCa [838].
6.3.2.4. Radiation therapy
6.3.2.4.1. Recommended IMRT/VMAT
Ultra-hypofractionated IMRT/IGRT or SBRT, using either 36.25 Gy (40 Gy to prostate) in 5 fx or 42.7 Gy in 7 fx can be offered to patients with NCCN favourable intermediate and good urinary function. Additional ADT is not required in GG2 disease [739]. Patients undergoing conventional or moderate hypofractionation and suitable for ADT can be treated with short-term ADT (four to six months) [839-841]. The RTOG 0815 RCT demonstrated improved BFSR, metastasis free and prostate CSS with the addition of six months ADT to dose escalated RT [756]. For adjuvant RT of the pelvic lymphatics (45-50 Gy) for NCCN unfavourable intermediate risk (cN0) see section 6.2.3.2.1. For patients unsuitable (e.g., due to co-morbidities) or unwilling to accept ADT (e.g., to preserve their sexual health) the recommended treatment is IMRT/VMAT (76–78 Gy or equivalent moderate HFX) or a combination of IMRT/VMAT and BT as described below. A secondary analysis of the PCS III trial has suggested that patients with NCCN favourable intermediate-risk disease (see Section 4.4) can safely omit ADT if their RT dose is 76 Gy, but this is based on an unplanned subgroup analysis and only 138 patients had favourable intermediate-risk disease. An individual discussion between the physician and the patient of the possible benefits and harms of omitting ADT in this group is essential [778].
6.3.2.4.2. Brachytherapy
Systematic review recommends LDR BT monotherapy can be offered to patients with NCCN favourable intermediate-risk disease and good urinary function (see section 4.4) [842]. Fractionated HDR BT as monotherapy can be offered to selected patients with intermediate-risk PCa although they should be informed that results are only available from small series in very experienced centres. Five-year PSA control rates over 90% are reported, with late grade 3+ GU toxicity rates < 5% and no, or very minimal, grade 3+ GI toxicity rates [796]. There are no direct data to inform on the use of ADT in this setting. Trimodality therapy with IMRT plus BT boost and short-term ADT can be considered for NCCN unfavourable intermediate-risk PCa (see section 4.4) but patients should be made aware that the potential improvements in biochemical control are accompanied with an increased risk of long-term urinary problems [785,787,792].
6.3.2.5. Other therapeutic options
6.3.2.5.1. Focal therapy
A prospective study on focal therapy using HIFU in patients with localised intermediate-risk disease was published but the data was derived from an uncontrolled single-arm case series [834]. There is a paucity of high-certainty data for any form of focal ablative therapy in the setting of intermediate-risk disease. Consequently, focal treatment cannot be considered as standard therapy for intermediate-risk patients and, if offered, it should only be in the setting of clinical trials or prospective registries [804].
6.3.2.5.2. Androgen deprivation therapy monotherapy
Data regarding the use of ADT monotherapy for intermediate-risk disease have been inferred indirectly from the EORTC 30891 trial, which was a RCT comparing deferred ADT vs. immediate ADT in 985 patients with T0–4 N0–2 M0 disease [843]. The trial showed a small, but statistically significant, difference in OS in favour of immediate ADT monotherapy but there was no significant difference in CSS, predominantly because the risk of cancer-specific mortality was low in patients with PSA < 8 ng/mL. Consequently, the use of ADT monotherapy for this group of patients is not considered as standard, even if they are not eligible for radical treatment.
6.3.2.6. Recommendations for the management of intermediate-risk disease*
| Recommendations | Strength rating |
| Expectant management | |
| Offer watchful waiting in asymptomatic patients with life expectancy < 10 years (based on comorbidities and age). | Strong |
| Offer active surveillance (AS) to selected patients with ISUP grade group 2 disease e.g., < 10% pattern 4, PSA < 10 ng/mL, ≤ cT2a, low disease extent on imaging and low extent of tumour in biopsies (≤ 3 positive cores with Gleason score 3+4 and ≤ 50% cancer involvement/core), or another single element of intermediate-risk disease with low disease extent on imaging and low biopsy extent, accepting the potential increased risk of metastatic progression. | Weak |
| Patients with ISUP grade group 3 disease should be excluded from AS protocols. | Strong |
| Re-classify patients with low-volume ISUP grade group 2 disease included in AS protocols, if repeat non-MRI-based systematic biopsies performed during monitoring reveal > 3 positive cores or maximum CI > 50%/core of ISUP grade group 2 disease. | Weak |
| Radical prostatectomy (RP) | |
| Offer RP to patients with a life expectancy of > 10 years. | Strong |
| Radical prostatectomy can be safely delayed for at least three months. | Weak |
| Offer nerve-sparing surgery to patients with a low risk of extra-capsular disease on that side. | Strong |
| Radiotherapeutic treatment | |
| Offer low-dose rate (LDR) brachytherapy to patients with good urinary function and NCCN favourable intermediate-risk disease. | Strong |
| Offer intensity-modulated radiotherapy (IMRT)/volumetric modulated arc therapy (VMAT) plus image-guided radiotherapy (IGRT), with a total dose of 76–78 Gy or moderate hypofractionation (60 Gy/20 fx in 4 weeks or 70 Gy/28 fx in 6 weeks), in combination with short-term androgen deprivation therapy (ADT) (four to six months). | Strong |
| Offer focal boosting to MRI-defined dominant intra-prostatic tumour when using conventionally fractionated IMRT/IGRT (1.8-2.0 Gy per fraction) ensuring that Organ at Risk constraints are not exceeded | Weak |
| Offer ultra-hypofractionated IMRT/IGRT or SBRT, using either 36.25 Gy (40 Gy to prostate) in 5 fx or 42.7 Gy in 7 fx delivered alternate days. | Weak |
| Offer LDR brachytherapy boost combined with IMRT/VMAT plus IGRT to patients with good urinary function and NCCN unfavourable intermediate-risk disease, in combination with short-term ADT (four to six months). | Weak |
| Offer high-dose rate (HDR) brachytherapy boost combined with IMRT/VMAT plus IGRT to patients with good urinary function and NCCN unfavourable intermediate-risk disease, in combination with short-term ADT (four to six months). | Weak |
| Other therapeutic options | |
| Only offer whole-gland ablative therapy (such as cryotherapy, high-intensity focused ultrasound, etc.) or focal ablative therapy within clinical trials or registries. | Strong |
| Do not offer ADT monotherapy to asymptomatic men not able to receive any local treatment. | Weak |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.3.3. Management of high-risk localised disease
Patients with high-risk PCa are at an increased risk of PSA failure, need for secondary therapy, metastatic progression and death from PCa. Nevertheless, not all high-risk PCa patients have a uniformly poor prognosis after RP [844]. When managed with non-curative intent, high-risk PCa is associated with 10-year and 15-year PCSM rates of 28.8 and 35.5%, respectively [845]. There is no consensus regarding the optimal treatment of men with high-risk PCa.
Some evidence suggests that radical treatment for high-risk PCa can be delayed up to three months after the diagnosis without any oncological consequences [846,847]. Systematic reviews suggest that there is a higher risk of biochemical recurrence and worse pathological outcomes when definitive treatment is given beyond a 6 to 9 months delay. However, there is no conclusive data regarding stronger endpoints (CSS or OS).
6.3.3.1. Radical prostatectomy
Provided that the tumour is not fixed to the pelvic wall or there is no invasion of the urethral sphincter, RP is a standard option in selected patients with a low tumour volume. Patients should be aware pre-operatively that surgery may be part of multi-modal treatment, with adjuvant or SRT or ADT. Neoadjuvant therapy using ADT is not indicated [848].
6.3.3.2. External beam radiation therapy
For high-risk localised PCa, a combined modality approach should be used consisting of IMRT/VMAT plus long-term ADT. The duration of ADT has to take into account PS, co-morbidities and the number of poor prognostic factors. It is important to recognise that in several studies EBRT plus short-term ADT did not improve OS in high-risk localised PCa and long-term ADT (at least two to three years) is currently recommended for these patients [748,749,757]. Moderate HFX is an option in high-risk patients with localised disease. The CHHiP study included 12% high-risk patients (n = 386) but limited entry to those with a PSA < 30 ng/mL and a Roach formula risk of SV involvement < 30% [728]. Patients were ineligible if they had both T3a tumours and ISUP grade group 4 or higher. The PCS-5 RCT used moderate HFX and elective nodal irradiation and efficiacy was equivalent in both groups [734,735].
6.3.3.2.1. Lymph node irradiation in cN0
There is no clear evidence for prophylactic irradiation of the pelvic LNs in intermediate- and high-risk disease. The long-term results of the NRG/RTOG 9413-trial which randomised intermediate-risk and high-risk localised PCa patients (1,322 cN0 patients were enrolled), showed that neoadjuvant HT plus whole pelvic RT improved PFS only compared with neoadjuvant ADT plus prostate RT and whole pelvic RT plus adjuvant ADT [849]. However, at the increased risk of ≥ grade 3 GI-toxicity.
A well-conducted single-centre RCT randomised 224 patients comparing prostate-only RT (PORT) vs. whole pelvic RT (WPRT) in localised high-risk- and locally-advanced tumours (cN0) with a risk of > 20% of positive nodes (Roach formula). With a median follow-up of 68 months there was a significant improvement of distant MFS (95.9% vs. 89.2%, HR: 0.35, p = 0.01) and DFS (89.5% vs.77.2%, p = 0.02). However, there was a significant higher rate of late GU ≥ 2 effects (17.7% vs. 7.5%, p = 0.02), the trial was relatively small in size with additional limitations and these findings are therefore insufficient to define a change in practice [850,851]. The benefits of pelvic nodal irradiation using IMRT/VMAT merit further investigation in large scale RCTs.
6.3.3.2.2. Brachytherapy boost
In men with NCCN unfavourable intermediate- or high-risk PCa, BT boost with supplemental EBRT and HT may be considered. See sections 6.2.3.4.1 and 6.2.3.4.2 for details on RCTs comparing EBRT alone and EBRT with LDR or HDR boost, respectively.
6.3.3.3. Recommendations for the management of high-risk localised disease*
| Recommendations | Strength rating |
| Expectant management | |
| Offer watchful waiting to asymptomatic patients with life expectancy < 10 years. | Strong |
| Radical prostatectomy (RP) | |
| Offer RP to selected patients as part of potential multi-modal therapy. | Strong |
| Extended pelvic lymph node dissection (PLND) | |
| In patients undergoing a lymph node dissection you should perform an extended PLND. | Strong |
| Do not perform a frozen section of nodes during RP to decide whether to proceed with, or abandon, the procedure. | Strong |
| Radiotherapeutic treatment | |
| Offer intensity-modulated radiotherapy (IMRT)/volumetric modulated arc therapy (VMAT) plus image-guided radiotherapy (IGRT), with a total dose of 76–78 Gy or moderate hypofractionation (60 Gy/20 fx in 4 weeks or 70 Gy/28 fx in 6 weeks), in combination with long-term androgen deprivation therapy (ADT) (two to three years). | Strong |
| Offer focal boosting to MRI-defined dominant intra-prostatic tumour when using normo-fractionated IMRT/IGRT (1.8-2.0 Gy per fraction) ensuring that Organ at Risk constraints are not exceeded. | Weak |
| Offer patients with good urinary function IMRT/VMAT plus IGRT with brachytherapy boost (either high-dose rate or low-dose rate), in combination with long-term ADT (two to three years). | Weak |
| Therapeutic options outside surgery or radiotherapy | |
| Do not offer either whole gland or focal therapy. | Strong |
| Only offer ADT monotherapy to those patients unwilling or unable to receive any form of local treatment if they have a prostate-specific antigen (PSA)-doubling time < 12 months, and either a PSA > 50 ng/mL or a poorly-differentiated tumour. | Strong |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.3.4. Treatment of locally-advanced PCa
In the absence of high-level evidence, a SR could not define the most optimal treatment option [852]. Randomised controlled trials are only available for EBRT. A local treatment combined with a systemic treatment provides the best outcome, provided the patient is fit enough to receive both. The initial results of the SCPG-15 trials suggested that randomisation between surgery and EBRT is feasible, but oncologic outcomes are awaited [853].
6.3.4.1. Radical prostatectomy
Surgery for locally-advanced disease as part of a multi-modal therapy has been reported [845,854,855]. However, the comparative oncological effectiveness of RP as part of a multi-modal treatment strategy vs. upfront EBRT with ADT for locally-advanced PCa remains unknown. A prospective phase III RCT (SPCG-15) comparing RP (with or without adjuvant or salvage EBRT) against primary EBRT and ADT among patients with locally-advanced (T3) disease is currently recruiting [856]. Data from retrospective case series demonstrated over 60% CSS at 15 years and over 75% OS at ten years [828,845,854,855,857-859]. For cT3b–T4 disease, PCa cohort studies showed 10-year CSS of over 87% and OS of 65% [829,860]. The indication for RP in all previously described stages assumes the absence of clinically detectable nodal involvement (cN0), based on conventional imaging. In case of suspected positive LNs during RP (initially considered cN0) the procedure should not be abandoned since RP may have a survival benefit in these patients. Intra-operative frozen section analysis is not justified in this case [462].
6.3.4.2. Treatment of cN1 M0 PCa
Lymph-node metastasized PCa is an entity where options for local therapy and systemic therapies overlap. Approximately 5% to 10% of newly diagnosed PCa patients have synchronous suspected pelvic nodal metastases on conventional imaging (CT/bone scan) without bone or visceral metastases (cN1 M0 stage).
6.3.4.2.1. Consideration of molecular imaging
Meta-analyses have shown that molecular imaging, such as PSMA-PET/CT, prior to primary treatment in advanced PCa detected disease outside the prostate in 32% of cases despite prior negative conventional imaging using bone scan and pelvic CT/MRI [453]. A RCT assessing PSMA-PET/CT as staging tool in high-risk PCa confirmed these findings and showed a 32% increase in accuracy compared with conventional imaging for the detection of pelvic nodal metastases [488]. Notably, more sensitive imaging also caused a stage shift with more cases classified as N1 on “molecular imaging” (miN1), but with, on average, lower nodal disease burden compared to cases classified as cN1.
The definition of miN1 is a subject of ongoing discussion given multiple guidelines exist as detection can be influenced by size of the lymph nodes and PSMA expression [104,861,862]. For patients with high or equivocal PSMA expression but normal size (< 10 mm), there is a lack of knowledge of the best treatment option and prospective data are encouraged [863].
6.3.4.2.2. Local treatment of cN1 M0 PCa
The management of cN1M0 PCa is historically based on long-term ADT combined with a local treatment with radiotherapy more commonly used than RP/pelvic nodal dissection. There is no randomised evidence available and the potential benefit of adding local treatment to ADT has been assessed in a non-randomised post-hoc analysis of STAMPEDE and retrospective studies summarised by Yaow et al. [864]. Pooled meta-analysis was performed for local treatment versus no local treatment (four studies, n = 4,597, local treatment n = 2,646) and showed improved estimated OS at all time points to ten years (OR: 1.49-1.81). The majority of patients underwent RT as local therapy. Assessment of RT vs. no local therapy (four studies, n=3,768) showed similar estimates for improvements in OS. Not included in this pooled analysis was STAMPEDE control arm data, that showed improvements in failure-free survival (adjusted HR: 0.48, 95% CI: 0.29-0.79) without severe toxicity [865] at median follow-up of seventeen months. Comparisons between local treatment modalities were limited by inclusion of retrospective studies, which fail to describe clearly how cN1 was defined.
Local treatment of cN1M0 disease in the era of taxane chemotherapy and ARPIs is under studied. Extended follow-up of STAMPEDE, reported as exploratory sub-analyses of patients who received docetaxel or control according to receipt of RT after median follow-up of 81.2 months, maintained failure-free survival benefit (HR: 0.68) in N+ patients but no prostate cancer specific survival (HR: 0.81) or OS (HR: 0.77) benefit was demonstrated [866]. Greatest benefits from RT were seen in the control (without docetaxel) group, as no significant benefits of RT receipt were seen in any category for the docetaxel group. Two RCTs from the STAMPEDE platform protocol reported a pre-planned meta-analysis of men with de novo high-risk/locally-advanced M0 disease, or relapse after primary curative therapy with high-risk features. Thirty-nine percent of patients (n=774) were N1 on conventional imaging [867]. Radiotherapy in addition to long-term ADT was administered in at least 71% of N1 patients. Data on survival according to whether RT was planned in N1 patients was not presented.
6.3.4.2.3. Systemic treatment of cN1 M0 PCa
The intensification of systemic treatment from initial ADT to other agents has been assessed within data from the STAMPEDE multi-arm RCT with a pre-planned meta-analysis in M0 patients. In cN1 M0 patients (39% of the cohort), improved metastasis free (HR: 0.49, 95% CI: 0.38-0.64) and overall (HR: 0.53, 95% CI: 0.39-0.70) survival was observed with intensification (abiraterone and/or enzalutamide) above standard of care (ADT +/- prostate radiotherapy in 85% of the whole cohort) in cN1M0 patients [867].
Considering intensification with docetaxel, exploratory sub-analyses of STAMPEDE non-metastatic (cN0/N1M0) patients who received docetaxel or control showed failure-free survival benefit (HR: 0.70, 95% CI: 0.56-0.88) but no metastatic progression-free (HR: 0.89) or OS (HR: 0.88) benefit [866]. Similar trends were observed in the N0 and N+ sub-groups. Radiotherapy was delivered to 77% of the cohort (see section 6.3.4.2). The AFU-GETUG 12 trial compared the impact of docetaxel plus estramustine in addition to ADT and 29% of included high-risk non-metastatic PCa patients had a nodal involvement (pN1) at randomisation [868]. Relapse-free survival rates were higher for cN1 patients receiving docetaxel plus estramustine but did not achieve statistical significance (HR: 0.66; 86 0.43–1.01). A meta-analysis of docetaxel trials in N0/N1-M0 patients concluded to an 8% four-year failure-free survival advantage for docetaxel compared with ADT alone without OS benefit (HR: 0.87, 95% CI: 0.69-1.09) [869].
Given the MFS and OS benefits observed in the overall population (see section 6.3.4.2), additional abiraterone (for 2 years) above standard of care (combined ADT for 3 years with prostate +/- WPRT) should be a SOC in cN1 patients.
Table 6.3.1: Selected studies assessing local treatment in (any cT) cN1 M0 prostate cancer patients
| Study | n | Design | Study period/ follow-up | Treatment arms | Effect on survival |
| ADT only | |||||
| Bryant, et al. 2018 [870] | 648 | Retrospective (National Veterans Affairs) | 2000-2015 61 mo. | ADT ± EBRT | Significant benefit for combined treatment only if PSA levels less than the median (26 ng/mL) All-cause mortality HR: 0.50 CSS, HR: 0.38 |
| Sarkar, et al. 2019 [871] | 741 | Retrospective (National Veterans Affairs) | 2000-2015 51 mo. | ADT ± local treatment (surgery or RT) | Significant benefit for RP All-cause mortality HR 0.36 CSS, HR: 0.32
No statistical difference for RP vs. RT (p ≥ 0.1) All-cause mortality HR: 047 CSS, HR: 0.88 |
Lin, et al. 2015 [872] | 983 before propensity score matching | Retrospective (NCDB) | 2004-2006 48 mo. | ADT ± EBRT | Significant benefit for combined treatment 5-yr. OS: 73% vs. 52% HR: 0.5 |
Tward, et al. 2013 [873]
| 1,100 | Retrospective (SEER) | 1988-2006 64 mo. | EBRT (n = 397) vs. no EBRT (n=703) No information on ADT) | Significant benefit for EBRT 5-yr. CSS: 78% vs. 71% HR: 0.66 5-yr. OS: 68% vs. 56%, HR: 0.70 |
| Rusthoven, et al. 2014 [874] | 796 | Retrospective (SEER) | 1995-2005 61 mo. | EBRT vs. no EBRT (no information on ADT) | Significant benefit for EBRT 10-yr. OS: 45% vs. 29% HR: 0.58 |
| Seisen, et al. 2018 [875] | 1,987 | Retrospective (NCDB) | 2003-2011 50 mo. | ADT ± local treatment (surgery or RT) | Significant benefit for combined treatment 5-yr. OS: 78.8% vs. 49.2% HR: 0.31 No difference between RP and RT |
| Chierigo, et al. 2022 [876] | 4,685 | Retrospective (SEER) | 2004–2016 | RP or RT (unknown ADT status) | Propensity score matching 5-yr OS: 84.6% (RP) vs. 75% (RT), HR 0.62, p < 0.001 5-yr CSS: 90.7% (RP) vs. 83% (RT), HR 0.62, p < 0.001 5-yr other cause mortality, 6.1% RP vs. 8.0% RT, HR 0.71, p = 0.04 |
| James, et al. 2016 [865] | 177 | Unplanned subgroup analysis RCT | 2005-2014
17 mo. | ADT ± EBRT (EBRT encouraged) | Significant benefit for combined treatment 5-yr. OS: 93% vs. 71% 2-yr. FFS: 81% vs. 53% FFS, HR: 0.48 |
| Elumalai, et al. 2023 [877] | 337 | Retrospective 4 centres UK | 2022-2019 | ADT +/- EBRT | Significant benefit for combined treatment 5-yr.OS: 87% vs. 56% HR: 0.27 5-yr. BPFS: 74.1% vs. 34.2% HR: 0.33 |
| Other systemic therapies | |||||
James, et al. 2022 [866] | 258 (N1 patients) | Planned subgroup analysis RCT | 2005-2018 81.2 mo | Standard of care (ADT +/- EBRT) +/- docetaxel
(EBRT planned for 55% SOC, 40% of docetaxel) | 5-year estimated Metastatic PFS (SOC + docetaxel vs SOC, HR: 0.79) OS (RT 78% vs no RT 71%, HR: 0.77)* CSS (RT 84% vs no RT 79%, HR: 0.81)* FFS (RT 51% vs no RT 36%, HR: 0.68)* *No stratification for docetaxel use |
Attard, et al. 2022 [867] | 774 (N1) | Planned subgroup analysis RCT | 2011-2016
72 mo | Standard of care (ADT +/- EBRT) +/- Abiraterone with or without enzalutamide (EBRT planned for 71% of N1 patients) | MFS (SOC + Abiraterone with or without enzalutamide vs SOC alone, HR: 0.49, 95% CI: 0.38-0.64)
OS (SOC + Abiraterone with or without enzalutamide vs SOC alone, HR: 0.53, 95% CI: 0.39-0.70) |
ADT = androgen deprivation therapy; CSS = cancer-specific survival; EBRT = external beam radiotherapy; FFS = failure-free survival; HR = hazard ratio; mo = months; n = number of patients; OS = overall survival; RP = radical prostatectomy; RT = radiotherapy; yr = year.
6.3.4.3. Options other than surgery or radiotherapy for primary treatment
6.3.4.3.1. Investigational therapies
Currently cryotherapy, HIFU or focal therapies have no place in the management of locally-advanced PCa.
6.3.4.3.2. Androgen deprivation therapy monotherapy
The deferred use of ADT as single treatment modality has been answered by the EORTC 30891 trial [843]. Nine hundred and eighty-five patients with T0–4 N0–2 M0 PCa received ADT alone, either immediately or after symptomatic progression or occurrence of serious complications. After a median follow-up of 12.8 years, the OS favoured immediate treatment (HR: 1.21, 95% CI: 1.05–1.39). Surprisingly, no different disease-free or symptom-free survival was observed, raising the question of survival benefit. In locally-advanced T3–T4 M0 HSPC unsuitable for surgery or RT, immediate ADT may only benefit patients with a PSA > 50 ng/mL and a PSA-DT < twelve months or those that are symptomatic [843,878]. The median time to start deferred treatment was seven years. In the deferred treatment arm 25.6% of patients died without needing treatment.
6.3.4.4. Recommendation for management of locally-advanced disease*
| Recommendations | Strength rating |
| Radical prostatectomy (RP) | |
| Offer RP to patients with cN0 disease as part of multi-modal therapy. | Weak |
| Extended pelvic lymph node dissection (PLND) | |
| In patients undergoing a lymph node dissection you should perform an extended PLND. | Strong |
| Radiotherapeutic treatments | |
| Offer patients with cN0 disease intensity-modulated radiation therapy (IMRT)/volumetric modulated arc therapy (VMAT) plus image-guide radiation therapy in combination with long-term androgen deprivation therapy (ADT). | Strong |
| Offer patients with cN0 disease and good urinary function, IMRT/VMAT plus IGRT with brachytherapy boost (either high-dose or low-dose rate), in combination with long-term ADT. | Weak |
| Offer long-term ADT for at least two years. | Strong |
| Offer IMRT/VMAT plus IGRT to the prostate in combination with long-term ADT and two years of abiraterone to cN0M0 patients with ≥ two high-risk factors (cT3-4, Gleason ≥ 8 or PSA ≥ 40 ng/mL). | Strong |
| Offer IMRT/VMAT plus IGRT to the prostate plus pelvis in combination with long-term ADT and two years of abiraterone to cN1M0 patients. | Strong |
| Other therapeutic options | |
| Do not offer whole gland treatment or focal treatment. | Strong |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.3.5. Adjuvant treatment after radical prostatectomy
6.3.5.1. Introduction
Adjuvant treatment is by definition additional to the primary or initial therapy with the aim of decreasing the risk of relapse, despite the apparent full control following surgery. A post-operative detectable PSA is an indication of persistent prostate cells (see section 6.3.6). All information listed below refers to patients with a postoperative undetectable PSA.
6.3.5.2. Risk factors for relapse
Patients with ISUP grade group > 2 in combination with EPE (pT3a) and particularly those with SV invasion (pT3b) and/or positive surgical margins are at high risk of progression, which can be as high as 50% after five years [879]. Irrespective of the pT stage, the number of removed nodes [880-887], tumour volume within the LNs and capsular perforation of the nodal metastases are predictors of early recurrence after RP for pN1 disease [888]. A LN density (defined as ‘the percentage of positive LNs in relation to the total number of analysed/removed LNs’) of over 20% was found to be associated with poor prognosis [889]. The number of involved nodes seems to be a major factor for predicting relapse [882,883,890]; the threshold considered is less than 3 positive nodes from an ePLND [458,882,890]. However, prospective data are needed before defining a definitive threshold value.
6.3.5.2.1. Biomarker-based risk stratification after radical prostatectomy
Biomarker-based risk stratification after radical prostatectomy
The Decipher® gene signature consists of a 22-gene panel representing multiple biological pathways and was developed to predict systemic progression after definitive treatment. A meta-analysis of five studies analysed the performance of the Decipher® Genomic Classifier (GC) test on men post-RP. The authors showed in multivariable analysis that Decipher® GC remained a statistically significant predictor of metastasis (HR: 1.30, 95% CI: 1.14–1.47, p < 0.001) per 0.1 unit increase in score and concluded that it can independently improve prognostication of patients post-RP within nearly all clinicopathologic, demographic, and treatment subgroups [891]. A SR of the evidence for the Decipher® GC has confirmed the clinical utility of this test in post-RP decision-making [892]. Further studies are needed to establish how to best incorporate Decipher® GC in clinical decision-making.
6.3.5.3. Immediate (adjuvant) post-operative external irradiation after RP (cN0 or pN0)
Four prospective RCTs have assessed the role of immediate post-operative RT (adjuvant RT [ART]) (undetectable PSA mostly defined as PSA < 0.1 ng/mL), demonstrating an advantage (endpoint, development of BCR) in high-risk patients (e.g., pT2 with positive surgical margins and ISUP grade group 3–5 or pT3/4 with- or without positive surgical margins and ISUP grade group 3–5) post-RP (Table 6.3.2). In the ARO 96-02 trial, 80% of the pT3/R1/GS 8–10 patients randomised to observation developed BCR within ten years [893]. It must be emphasised that PSA was undetectable at inclusion only in the ARO 96-02 trial which presents a major limitation interpreting these findings as patients with a detectable PSA would now be considered for salvage therapy rather than ART [893].
6.3.5.4. Comparison of adjuvant and salvage radiotherapy
Two retrospective matched studies (510 and 149 patients receiving ART) failed to show an advantage for MFS [894,895]. However, both studies were underpowered for high-risk patients (pT3b/R1/ISUP grade group 4–5 PCa). In contrast to these studies, a propensity score-matched retrospective analysis of two cohorts of 366 pT3 and/or R1 patients found that compared to SRT at a PSA between 0.1 and 0.5 ng/mL, ART given at an undetectable PSA (< 0.1 ng/mL) improved all three endpoints; BCR, MFS, and OS [896].
Both approaches (ART and early SRT) together with the efficacy of adjuvant ADT are compared in three prospective RCTs: the Medical Research Council (MRC) Radiotherapy and Androgen Deprivation In Combination After Local Surgery (RADICALS) trial [897], the Trans-Tasman Oncology Group (TROG) Radiotherapy Adjuvant Versus Early Salvage (RAVES) trial [898], and the Groupe d’Etude des Tumeurs Uro- Genitales (GETUG-AFU 17) trial [899]. In addition, a pre-planned meta-analysis of all three trials has been published (Table 6.3.3) [900].
Two trials closed early after randomising 333/470 patients (RAVES) and 424/718 (GETUG-AFU-17) patients. RADICALS-RT included 1,396 patients, ninty-three percent (648/697) in the ART – Group. At the time of the ten year analysis 39% (270/699) of the Savage-RT-Policy Group started SRT with a median pre-SRT PSA-level of 0.2 ng/ml. With the option of subsequent inclusion in RADICALS-HT; 154/649 (24%) of patients starting in the adjuvant RT group also received neoadjuvant or adjuvant HT; 90 patients for six months/45 for 2 years/19 patients outside RADICALS-HT. From the SRT group, 61/228 (27%) received neoadjuvant or adjuvant HT for six months (n = 33) and two years (n = 13). Fifteen of these patients were treated outside the trial [897]. All men in the GETUG-AFU-17 trial (n = 424) received six months of HT. All together, 684 out of 2,153 patients received additional ADT for at least six months across both trials [900]. Radiotherapy to the pelvic lymphatics was allowed in the GETUG-AFU and in the RADICALS-RT trials.
The primary endpoint for RAVES and GETUG-AFU 17 was biochemical PFS, and for RADICALS-RT MFS. So far only RADICALS-RT have reported the ten year primary endpoint data [901]. With a median follow up of 7.8 years the 10 year FFDM was 93% (ART) versus 90% (SRT)(HR 0.68, p=0.095) although based upon just 80 events in 1,396 patients. BPFS and OS also showed no significant difference (Table 6.3.3). With a median follow-up between 4.9 years and 6.25 years in the ARTISTIC-Metaanalysis there was no statistically significant difference for biochemical PFS for both treatments in all three trials (see Table 6.2.3). Additionally, there was a significant lower rate of grade ≥ 2 GU late side effects and grade 3–4 urethral strictures in favour of early SRT; which may also be caused by the low number of patients with PSA-progression and subsequent need for early SRT at the time of analysis (40% of patients) [900].It should be noted, the side-effect profile may have been impacted with a larger proportion of ART patients receiving treatment with older 3D-treatment planning techniques as compared to SRT patients (GETUG-AFU 17: ART, 69% 3D vs. 46% SRT) and patients treated more recently were more likely to undergo IMRT techniques with a proven lower rate of late side effects [702]. However, on the basis of these three trials patients with ‘low-risk factors’ of biochemical progression after RP should be closely followed up with ultra-sensitive assays and SRT should be discussed if needed as soon as PSA starts to rise, which has to be confirmed by a second PSA measurement.
The proportion of patients with adverse pathology at RP (ISUP GG 4–5 and pT3 with or without positive margins) in all three trials was low (between 10–20%) and therefore even the meta-analysis may be underpowered to show an outcome in favour of SRT [900]. The subset analysis of this primary endpoint based on the prerandomization strata (i.e. the high risk features Gleson 8-10 vs. < 7 and pT3b-4 vs. <=pT3a) is still awaited to inform if these high risk groups benefit from ART compared with SRT. However, a retrospective multi-centre study comparing ART and SRT in 26,118 patients of whom 2,424 had high-risk features (pN1 or ISUP GG 4–5 and pT3/4-tumours) after RP [902] does support ART. With a median follow-up of 8.2 years and after excluding men with persistent PSA after RP, ART when compared with early SRT showed a significantly lower acute mortality risk (p = 0.02, HR: 0.33). Therefore, ART remains a recommended treatment option in highly selected patients with adverse pathology (‘high-risk patients’) i.e. ISUP grade group 4–5 and pT3 with or without positive margins [903,904].
In conclusion, the vast majority of patients with an undetectable PSA (<0.1 ng/ml) after RP do not need ART. However, in patients with high risk factors (pT3/4 and ISUP 4-5) ART to the prostatic bed should be given as they were underrepresented in RADICALS and in the metaanalysis too [897-900] on the one hand and the proven effect in RCT‘s on the other hand [893,905,906].
Table 6.3.2: Overview of all four randomised trials for adjuvant surgical bed radiation therapy after RP* (without ADT)
| Overview of all four randomised trials for adjuvant surgical bed radiation therapy after RP | |||||||
| Study | n | Inclusion criteria | Randomisation | Definition of BCR PSA (ng/mL) | Median FU (mo) | Biochemical Progression-free survival | Overall survival |
SWOG 8794 2009 [905] | 431 | pT3 cN0 ± involved SM | 60-64 Gy vs. observation | > 0.4 | 152 | 10 yr.: 53% vs. 30% (p < 0.05) | 10 yr.: 74% vs. 66% Median time: 15.2 vs. 13.3 yr., p = 0.023 |
EORTC 22911 2012 [906] | 1,005 | pT3 ± involved SM pN0 pT2 involved SM pN0 | 60 Gy vs. observation | > 0.2 | 127 | 10 yr.: 60.6% vs. 41% (p < 0.001) | 81% vs. 77% n.s. |
ARO 96-02 2014 [893] | 388 | pT3 (± involved SM) pN0 PSA post-RP undetectable | 60 Gy vs. observation | > 0.05 + confirmation | 112 | 10 yr.: 56% vs. 35% (p = 0.0001) | 10 yr.: 82% vs. 86% n.s. |
FinnProstate Group 2019 [907] | 250 | pT2,R1/ pT3a | 66.6 Gy vs. observation (+ SRT) | > 0.4 (in 2 successive measure- ments) | 112 vs. 103 (patients alive) | 10 yr.: 82% vs. 61% p < 0.001 | 10 yr.: 92% vs. 87% n.s. |
*See Section 6.3.5.1 for delayed (salvage) post-radical prostatectomy external irradiation.
BCR = biochemical recurrence; FU = follow-up; mo = months; n = number of patients; n.s. = not significant; OS = overall survival; PSA = prostate-specific antigen; RP = radical prostatectomy; SM = surgical margin;SRT = salvage radiotherapy.
Table 6.3.3: Overview of all three randomised trials and one meta-analysis for patients treated with adjuvant vs. early salvage RT after RP
| Overview of all three randomised trials and one meta-analysis | ||||||||
| Study | n | Inclusion criteria | Randomisation | Definition of BCR PSA (ng/mL) | Median FU (yr) | BPFS | OS or MFS | Side effects |
RAVES TROG 08.03/ ANZUP 2020 [898] | 333 target was 470 early closed | pT3a/pT3b any T - SM+ PSA post-RP: | 64 Gy ART PSA: 64 Gy early SRT at PSA med. pre-SRT: n.r. | > 0.4 post RT | 6.1 | 5 yr.: 86% vs. 87% (p > 0.05) | n.r. | LT grade ≥ GU: 70% vs. 54% (p = 0.002) |
| RADICALS-RT [897] | 1,396 | pT3a/ pT3b/pT4 PSA any T, SM+ Gleason 7-10 PSA post-RP: < 0.2 ng/mL | 52.5 Gy (20 Fx) or 66 Gy (33 Fx) ART early SRT identical at PSA > 0.1 med.pre-SRT: | > 0.4 or 2 at any time | 4.9 | 5 yr.: 85% vs. 88% (p = 0.56) | n.r. | Self-reported urinary incontinence 1 yr: 4.8 vs. 4 (p = 0.023) urethral stricture grade 3/4 2 yr: 6% vs. 4% (p = 0.02) |
| GETUG-AFU 17 2020 [899] | 424 target was 718 early closed | pT3a/pT3b/pT4a and SM + PSA post-RP: < 0.1 ng/mL | 66 Gy (ART) vs. 66 Gy early SRT at PSA 0.1 both groups: 6 mo. LHRH-A med. pre-SRT 0.24 | > 0.4 | 6.25 | 5 yr: 92% vs. 90% (p = 0.42) | n.r. | LT grade > 2 GU 27% vs. 7% (p < 0.001) ED: 28% vs. 8% (p < 0.001) |
| ARTISTIC 2020 [900] | 2,153 | see above | see above | see above | 4.5 | 5 yr.: 89% vs. 88% p = 0.7 | n.r. | n.r. |
ART = adjuvant radiotherapy; BCR = biochemical recurrence; BPFS = biochemical progression-free survival; ED = erectile dysfunction; FU = follow-up; Fx = fraction; GU = genito-urinary; LHRH = luteinising hormonereleasing hormone; LT = late toxicity; mo = months; med = median; MFS = metastasis-free survival; n.r. = not reported;OS = overall survival; PSA = prostate-specific antigen; RP = radical prostatectomy; RT = radiotherapy;SRT = salvage radiotherapy; + = positive; yr = year.
6.3.5.5. Adjuvant systemic therapy in N0 disease
The TAX3501 trial comparing the role of leuprolide (18 months) with and without docetaxel (6 cycles) ended prematurely due to poor accrual. A phase III RCT comparing adjuvant docetaxel against surveillance after RP for locally-advanced PCa showed that adjuvant docetaxel did not confer any oncological benefit [908]. Consequently, adjuvant chemotherapy after RP should only be considered in a clinical trial [909].
6.3.5.6. Adjuvant treatment in pN1 disease
6.3.5.6.1. Adjuvant androgen ablation alone
The combination of RP and early adjuvant HT in pN+ PCa has been shown to achieve a ten-year CSS rate of 80% and has been shown to significantly improve CSS and OS in prospective RCTs [910,911]. However, these trials included mostly patients with high-volume nodal disease and multiple adverse tumour characteristics and these findings may not apply to men with less extensive nodal metastases.
6.3.5.6.2. Adjuvant radiotherapy combined with ADT in pN1 disease
In a retrospective multi-centre cohort study, maximal local control with RT to the prostatic fossa appeared to be beneficial in PCa patients with pN1 after RP, treated ‘adjuvantly‘ with continuous ADT (within six months after surgery irrespective of PSA). The beneficial impact of adjuvant RT on survival in patients with pN1 PCa was highly influenced by tumour characteristics. Men with low-volume nodal disease (< 3 LNs), ISUP grade group 2–5 and pT3–4 or R1, as well as men with 3 to 4 positive nodes were more likely to benefit from RT after surgery, while the other subgroups did not [912]. In contrast, a retrospective multi-centre study including 1,614 patients and a median follow-up of 7.02 years assessed ART + ADT. Adjuvant RT compared to SRT was associated with a decreased all-cause mortality and this reduction increased with each additional positive pelvic LN, from the first one on and the highest effect was for more than 3 positive nodes [913]. These data are in agreement with a US National Cancer Database analysis based on 5,498 patients [914]. Another US National Cancer Database study including 8,074 pN1 patients reports improved OS after ADT plus EBRT (including pelvic LNs) vs. observation and vs. ADT alone in all men with single or multiple adverse pathological features. Men without any adverse pathological features did not benefit from immediate adjuvant therapy [915].
In a SR of the literature, RT with or without ADT was associated with improved survival in men with locally-advanced disease and a higher number of positive nodes [916]. Radiotherapy to the pelvic lymphatics and the prostate fossa plus long-term ADT can be offered to patients with pN1 disease [912,917]. However, the optimal duration of ADT is still unkown.
6.3.5.6.3. Observation of pN1 patients after radical prostatectomy and extended lymph node dissection
Several retrospective studies and a SR addressed the management of patients with pN1 PCa at RP [890,912,916-918]. A subset of patients with limited nodal disease (1–2 positive LNs) showed favourable oncological outcomes and did not require additional treatment.
An analysis of 209 pN1 patients with one or two positive LNs at RP showed that 37% remained metastasis-free without need of salvage treatment at a median follow-up of 60.2 months [918]. Touijer et al., reported their results of 369 pN1-positive patients (40 with and 329 without adjuvant treatment) and showed that higher pathologic grade group and > 3 positive LNs were significantly associated with an increased risk of BCR on multivariable analysis [890]. Biochemical-free survival rates in pN1 patients without adjuvant treatment ranged from 43% at four years to 28% at ten years [916]. Reported CSS rates were 78% at five years and 72% at ten years. The majority of these patients were managed with initial observation after surgery, had favourable disease characteristics, and 63% had only one positive node [916]. Initial observation followed by early salvage treatment at the time of recurrence may represent a safe option in selected patients with a low disease burden [916].
6.3.5.7. Recommendations for adjuvant treatment for pN0 and pN1 disease after radical prostatectomy*
| Recommendations | Strength rating |
| Do not prescribe adjuvant androgen deprivation therapy (ADT) to pN0 patients. | Strong |
| In pN0 patients with ISUP grade group 4–5 and pT3 ± positive margins, offer adjuvant intensity-modulated radiation therapy (IMRT)/volumetric modulated arc therapy (VMAT) plus image-guided radiation therapy (IGRT). | Strong |
In pN1 patients, after an extended lymph node dissection, discuss three management options, based on nodal involvement characteristics: 1. Offer adjuvant ADT. 2. Offer adjuvant ADT with additional IMRT/VMAT plus IGRT. 3. Offer observation (expectant management) to a patient after ePLND and ≤ 2 nodes and an undetectable PSA. | Weak |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.3.6. Persistent PSA after radical prostatectomy
Between five and 20% of men continue to have detectable or persistent PSA after RP (when defined in the majority of studies as detectable post-RP PSA of ≥ 0.1 ng/mL within 4 to 8 weeks of surgery) [919,920]. Improvements in the sensitivity of PSA assays now allow for the detection of PSA at much lower levels. It may result from persistent local disease, pre-existing metastases or residual benign prostate tissue.
6.3.6.1. Natural history of persistently elevated PSA after RP
Two SRs addressing persistent PSA confirmed a strong correlation of PSA persistence with poor oncologic outcomes [919,920]. A meta-analysis of consecutive patient cohorts reported that persistent PSA was more likely when risk factors, such as high D’Amico risk, Gleason score ≥ 8, pT stage ≥ 8 and presence of extraprostatic extension, seminal vesicle invasion, lymph node involvement, positive margin, were present [921]. Salvage radiotherapy was also more likely to be given to patients with persistent PSA. Cribiform pattern or intraductal carcinoma have also been associated with persistent PSA [922].
Considering oncological outcomes, patients with persistent PSA (≥ 0.1 ng/mL) had worse biochemical recurrence-free (HR: 3.86, 95% CI: 2.4 – 6.22), metastasis-free (HR 3.6, 95% CI 2.94-4.42) and prostate cancer-specific (HR: 3.54, 95% CI: 2.4-5.22) survival on meta-analysis of retrospective cohorts [921]. The largest study by Preisser et al. (n = 11,605) showed that persistent PSA is prognostic of an increased risk of metastasis and death [923] [864]. At fifteen years after RP, MFS rates, OS and CSS rates were 53.0 vs. 93.2% (p < 0.001), 64.7 vs. 81.2% (p < 0.001) and 75.5 vs. 96.2% (p < 0.001) for persistent vs. undetectable PSA, respectively. The median follow-up was 61.8 months for patients with undetectable PSA vs. 46.4 months for patients with persistent PSA. In multivariable Cox regression models, persistent PSA represented an independent predictor for metastasis (HR: 3.59, p < 0.001), death (HR: 1.86, p < 0.001) and cancer-specific death (HR: 3.15, p < 0.001), similarly for pathologic stage pT3b and ISUP Grade Group 3-5.
However, not all patients with persistent PSA after RP experience disease recurrence. Xiang et al., showed a 50% five-year BCR-free survival in men who had a persistent PSA level > 0.1 but ≤ 0.2 ng/mL at six to eight weeks after RP [924]. Rogers et al., assessed the clinical outcome of 160 men with a persistently detectable PSA level after RP [925]. No patient received adjuvant therapy before documented metastasis. In their study, 38% of patients had no evidence of metastases for ≥ 7 years while 32% of the patients were reported to develop metastases within three years. Most patients had Gleason score 7 (44%) or ≥ 8 (49%). In multi-variable analysis, the PSA slope ≥ 0.05 after RP (as calculated using PSA levels three to twelve months after surgery; HR: 2.7) and pathological ISUP GG (≥ 3 vs. ≤ 2; HR: 1.8) were significantly associated with the development of distant metastases among patients with persistent PSA. Prostate-specific antigen slope is more commonly reported as PSA doubling time (calculated by log [PSA slope]) [926].
6.3.6.2. Imaging in patients with persistently elevated PSA after RP
PSMA PET/CT is known to have superior detection efficiency, however dedicated studies for patients with persistently elevated PSA after RP are limited compared to studies inclusive of patients with BCR with/without persistent PSA.
Considering the persistent PSA group, a multi-centre retrospective study included 191 patients with persistently elevated PSA after RP and 68Ga-PSMA-PET/CT was positive in 68%, of which 35% had disease confined to the pelvis (obturator, presacral/mesorectal most common) and 33% had distant metastases [927]. A subgroup analysis of 33 patients with pre- and post-RP imaging showed PET-persistence in 45%, new lesions in 24% and negative post-RP PET in 30%. Another retrospective study included 150 patients with persistent PSA after RARP who were re-staged with both 68Ga-PSMA and 18F-DCFPyL PSMA. The authors found that in the presence of persistent PSA the majority of patients already had involved pelvic LNs (33%) or distant metastases (26%) which would support a role of PSMA PET/CT imaging in guiding (salvage) treatment strategies [928]. Schmidt-Hegemann et al., studied 129 patients who had either persistent PSA (52%) or BCR (48%) after RP, showing that men with a persistent PSA had significantly more pelvic nodal involvement on PSMA PET/CT than those with an initially undetectable PSA [929].
Therefore, PSMA PET serves to identify sites of remnant disease in patients with persistent PSA after RP. At present there is uncertainty regarding the best treatment if PSMA PET/CT shows metastatic disease outside the pelvis.
6.3.6.3. Management options for patients with persistent PSA
6.3.6.3.1. Comparison with biochemical recurrence (BCR)
It is clear that persistent PSA after RP is a poor prognostic indicator, likely representative of low volume synchronous metastatic disease rather than metachronous disease like in biochemical recurrence. A retrospective analysis of the RTOG 9601 trial of SRT +/- ADT (bicalutamide) for biochemical failure after RP considered patients with persistent PSA (n=90) or BCR (n=670) as the cause for biochemical failure and, following statistical adjustment, showed higher 10-year metastatic progression rate (28.6% vs. 10.1%, p < 0.0001), numerically higher 10-year overall mortality rate (24.9% vs. 11.9%, p = 0.03) and higher local progression rate (3.2% vs. 1.4%, p = 0.0001) [930]. In the ARO 96-02, a prospective RCT, 74 patients with PSA persistence (20%) received immediate SRT only (66 Gy per protocol [arm C]). The 10-year clinical relapse-free survival was 63% and showed worse 10-year metastasis-free survival (67% vs 83%) and OS (68% vs 84%) than BCR patients [931]. Therefore, it is likely that outcomes are worse than for men with persistent PSA than those experiencing BCR [932]. Indeed, studies investigating PSA persistence were excluded from the EAU Guidelines Biochemical Recurrence risk groups [933].
6.3.6.3.2. Post-operative RT
The benefit of post-operative RT (adjuvant or salvage) in patients with persistent PSA remains unclear due to a lack of RCTs. Ploussard et al., reported following SR that SRT was associated with improved survival outcomes, although the available evidence is of low quality [920].
Preisser et al., compared oncological outcomes of patients with persistent PSA who received SRT vs. those who did not [923]. In the subgroup of patients with persistent PSA, after 1:1 propensity score matching between patients with SRT vs. no RT, OS rates at ten years after RP were 86.6 vs. 72.6% in the entire cohort (p < 0.01), 86.3 vs. 60.0% in patients with positive surgical margin (p = 0.02), 77.8 vs. 49.0% in pT3b disease (p < 0.001), 79.3 vs. 55.8% in ISUP grade group 3-5 disease (p < 0.01) and 87.4 vs. 50.5% in pN1 disease (p < 0.01), respectively. Moreover, CSS rates for patients who underwent SRT vs. no RT at ten years after RP were 93.7 vs. 81.6% in the entire cohort (p < 0.01), 90.8 vs. 69.7% in patients with positive surgical margin (p = 0.04), 82.7 vs. 55.3% in pT3b disease (p < 0.01), 85.4 vs. 69.7% in ISUP grade group 3-5 disease (p < 0.01) and 96.2 vs. 55.8% in pN1 disease (p < 0.01), respectively. In multi-variable models, after 1:1 propensity score matching, SRT was associated with lower risk of death (HR: 0.42, p = 0.02) and lower cancer-specific death (HR: 0.29, p = 0.03). SRT dose was 46Gy for the 54% of patients with available data, but SRT field and ADT use was unavailable. The benefit of SRT in improving MFS (HR 0.39, p = 0.001), CSS (HR 0.34, p = 0.03) and OS (HR 0.24, p = 0.001) were also observed in a retrospective analysis of 3,409 patients who underwent RP (9.2% persistent PSA, median follow-up 4.5 years) by Özman et al. [934].
It is clear from a number of studies that poor outcomes are driven by the level of pre-RT PSA, the presence of ISUP grade group ≥ 4 in the RP histology and pT3b disease [931,935-939] [878-883]. Fossati et al., suggested that only men with a persistent PSA after RP and ISUP grade group ≤ 3 benefit significantly [940], similarly supported by Özman et al. where positive margins, higher T-stage, pN1 and lower ISUP Grade group were most likely to benefit from SRT, although this was not supported by Preisser et al. [923,934].
The current data do not allow clear treatment recommendations. However, these benefits in the SRT + ADT group (compared to ADT alone) were associated with higher incidence of bowel symptoms (34 vs. 19%, p = 0.01) and bothersome incontinence if given within 6 months of surgery (p < 0.001) [934].
6.3.6.3.3. Multimodal therapy (ADT with post-operative RT)
Addition of ADT may improve PFS [935]. Choo et al., prospectively studied the addition of two-year ADT to immediate RT to the prostate bed in patients with pT3 and/or positive surgical margins after RP [935]. Twenty-nine of the 78 included patients had persistently detectable post-operative PSA. The relapse-free rate was 85% at five years and 68% at seven years, which was superior to the five-year progression-free estimates of 74% and 61% in the post-operative RT arms of the EORTC and the SWOG studies, respectively, which included patients with undetectable PSA after RP [905,906]. Patients with persistently detectable post-operative PSA comprised approximately 50% and 12%, respectively, of the study cohorts in the EORTC and the SWOG studies.
A multi-centre, retrospective study from Japan considered 383 patients with pN1 and persistent PSA after RP and reported that the addition of SRT (median 66Gy; prostate bed with pelvis 67%, prostate bed alone 24%) to ADT showed better castration resistance-free (5-year p < 0.001, 10-year p = 0.02) and metastasis-free (5-year p < 0.001, 10-year p = 0.15) but not OS than ADT alone in patients with pre-treatment PSA ≥ 0.52 ug/L [941]. Similar benefits have been reported for SRT with ADT compared to ADT alone in single centre retrospective studies [942,943].
The phase 2 GETUG-22 trial comparing RT (46Gy pelvis with 66Gy prostate bed boost) with RT plus short-term ADT for post-RP PSA persistence (0.2–2.0 ng/mL) in 125 patients reported good tolerability of the combined treatment. The oncological endpoints are yet to be published [944].
6.3.6.4. Conclusion
The available data suggest that patients with PSA persistence after RP have worse prostate-cancer outcomes and serve to benefit most from early aggressive multimodality treatment, however, the lack of prospective RCTs makes firm recommendations difficult.
6.3.6.5. Recommendations for the management of persistent PSA after radical prostatectomy
| Recommendations | Strength rating |
| Offer a prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) scan to men with a persistent prostate-specific antigen (PSA) and rising if the results will influence subsequent treatment decisions. | Weak |
| Treat men with persistent PSA and no evidence of distant metastatic disease with salvage radiotherapy and additional hormonal therapy. | Weak |
6.4. Management of PSA-only recurrence after treatment with curative intent
Follow-up will be addressed in Chapter 7 and is not discussed in this section.
6.4.1. Background
Between 27% and 53% of all patients undergoing RP or RT develop a rising PSA (PSA recurrence). Whilst metastatic progression is universally preceded by rising PSA levels, physicians must inform the patient that the natural history of PSA-only recurrence may be prolonged and that a measurable PSA may not necessarily lead to clinically apparent metastatic disease. Physicians treating patients with PSA-only recurrence face a difficult set of decisions in attempting to delay the onset of metastatic disease and death while avoiding overtreating patients whose disease may never affect their OS or QoL. It should be emphasised that the treatment recommendations for these patients should be given after discussion in a multidisciplinary team.
6.4.1.1. PSA velocity and doubling time
Various PSA kinetics definitions have been proposed with different methods of calculation (log transformed or not) and eligible PSAs:
- PSA velocity (PSAV): absolute annual increase in serum PSA (ng/mL/year);
- PSA doubling time (PSA-DT): which measures the exponential increase in serum PSA over time.
Prostate-specific antigen velocity is more simple to calculate by subtracting the initial value from the final value, dividing by time. However, by ignoring middle values, not all PSA values are accurately taken into account.
Prostate-specific antigen-DT is calculated assuming an exponential rise in serum PSA. The formula takes into account the natural logarithm of 2 divided by the slope obtained from fitting a linear regression of the natural log of PSA over time [945]. However, many different PSA-DT calculations have been assessed according to the mathematical formula used and to the included PSA values (number, time period, intervals) [946]. For example, the ‘MSKCC’ method calculates a regression slope integrating all PSA values. Other methods transform PSA before calculating the slope and do not include all PSA values (different time frames and minimal intervals) [947]. O’Brien and colleagues identified more than 20 different definitions of PSAV and PSA-DT and demonstrated that obtained values could vary widely between definitions [947].
However, some rules can be considered for PSA-DT calculation [945]:
- At least three PSA measurements are required;
- A minimum time period between measurements (4 weeks) is preferable due to potential statistical ‘noise’ when PSA values are obtained too close together (this statement can be reconsidered in case of very active disease);
- All included PSA values should be obtained within the past twelve months at most, to reflect the current disease activity;
- PSA-DT is often mentioned in months, or in weeks in very active disease.
These measurements do not provide additional information compared with PSA alone [540,947-949]. In the post-local therapy relapse setting, PSA-DT has been correlated with distant progression and with poorer outcomes after salvage treatments [950,951]. Prostate-specific antigen-DT has been linked with metastasis-free- and OS in non-metastatic CRPC (nmCRPC) and identifies patients with high-risk nmCRPC who benefit from intensified therapy (PSA-DT threshold < ten months) [952].
6.4.2. Controversies in the definitions of clinically relevant PSA relapse
The PSA level that defines treatment failure depends on the primary treatment. Patients with rising PSA after RP or primary RT have different risks of subsequent symptomatic metastatic disease based on various parameters, including the PSA level. Therefore, physicians should carefully interpret BCR endpoints when comparing treatments. Clinicians should interpret a PSA rise in light of the EAU BCR risk groups [933].
After RP, the threshold that best predicts further metastases is a PSA > 0.4 ng/mL and rising [953]. However, with access to ultra-sensitive PSA testing, a rising PSA much below this level will be a cause for concern for patients. After primary RT, with or without short-term hormonal manipulation, the RTOG-ASTRO Phoenix Consensus Conference definition of PSA failure (with an accuracy of > 80% for clinical failure) is ‘any PSA increase > 2 ng/mL higher than the PSA nadir value, regardless of the serum concentration of the nadir’ [954]. After HIFU or cryotherapy no endpoints have been validated against clinical progression or survival; therefore, it is not possible to give a firm recommendation of an acceptable PSA threshold after these alternative local treatments [933].
6.4.3. Natural history of biochemical recurrence
Once a PSA recurrence has been diagnosed, it is important to determine whether the recurrence has developed at local or distant sites. A SR and meta-analysis investigated the impact of BCR on clinical endpoints and concluded that patients experiencing BCR are at an increased risk of developing distant metastases, PCa-specific and overall mortality [933]. However, the effect size of BCR as a risk factor for mortality is highly variable. After primary RP its impact ranges from HR 1.03 (95% CI: 1.004–1.06) to HR 2.32 (95% CI: 1.45–3.71) [955,956]. After primary RT, OS rates are approximately 20% lower at eight to ten years follow-up even in men with minimal co-morbidity [957,958]. Still, the variability in reported effect sizes of BCR remains high and suggests that only certain patient subgroups with BCR might be at an increased risk of mortality.
The risk of subsequent metastases, PCa-specific- and overall mortality may be predicted by the initial clinical and pathologic factors (e.g., T-category, PSA, ISUP grade group) and PSA kinetics (PSA-DT and interval to PSA failure), which was further investigated by the SR [933].
For patients with BCR after RP, the following outcomes were found to be associated with significant prognostic factors:
- distant metastatic recurrence: positive surgical margins, high RP specimen pathological ISUP GG, high pT category, short PSA-DT, high pre-SRT PSA;
- prostate-cancer-specific mortality: high RP specimen pathological ISUP grade group, short interval to biochemical failure as defined by investigators, short PSA-DT;
- overall mortality: high RP specimen pathological ISUP grade group, short interval to biochemical failure, high PSA-DT.
For patients with BCR after RT, the corresponding outcomes are:
- distant metastatic recurrence: high biopsy ISUP grade group, high cT category, short interval to biochemical failure;
- prostate-cancer-specific mortality: short interval to biochemical failure;
- overall mortality: high age, high biopsy ISUP grade group, short interval to biochemical failure, high initial (pretreatment) PSA.
Based on this meta-analysis, proposal is to stratify patients into two risk categories since not all patients with BCR will have similar outcomes (see Table 6.4.1). The stratification into ‘EAU Low-Risk’ or ‘EAU High-Risk’ BCR after RP has been validated in a European cohort [959].
Table 6.4.1: EAU risk categories for patients developing biochemical recurrence
| EAU Low Risk BCR | EAU High Risk BCR | |
| After RP | PSA-DT > 1 yr AND pathological ISUP grade group < 4 | PSA-DT ≤ 1 yr OR pathological ISUP grade group 4–5 |
| After RT | interval to biochemical failure > 18 mo AND biopsy ISUP grade group < 4 | interval to biochemical failure ≤ 18 mo OR biopsy ISUP grade group 4–5 |
6.4.4. The role of imaging in PSA-only recurrence
Imaging is only of value if it leads to a treatment change which results in an improved outcome. In practice, however, there are very limited data available regarding the outcome’s consequent on imaging at recurrence.
6.4.4.1. Assessment of metastases (including nodal)
6.4.4.1.1. Bone scan and abdominopelvic CT
Because BCR after RP or RT precedes clinical metastases by seven to eight years on average [884,960], the diagnostic yield of conventional imaging techniques (bone scan and abdominopelvic CT) is low in asymptomatic patients [961]. In men with PSA-only recurrence after RP the probability of a positive bone scan is < 5%, when the PSA level is < 7 ng/mL [962,963]. Only 11–14% of patients with BCR after RP have a positive CT [962]. In a series of 132 men with BCR after RP the mean PSA level and PSA velocity associated with a positive CT were 27.4 ng/mL and 1.8 ng/mL/month, respectively [964].
6.4.4.1.2. Choline PET/CT
In two different meta-analyses the combined sensitivities and specificities of choline PET/CT for all sites of recurrence in patients with BCR were 86–89% and 89–93%, respectively [965,966]. Choline PET/CT may detect multiple bone metastases in patients showing a single metastasis on bone scan [967] and may be positive for bone metastases in up to 15% of patients with BCR after RP and negative bone scan [968]. The specificity of choline PET/CT is also higher than bone scan, with fewer false positive and indeterminate findings [473]. Detection of LN metastases using choline PET/CT remains limited by the relatively poor sensitivity of the technique. Choline PET/CT sensitivity is strongly dependent on the PSA level and kinetics [483,969,970]. In patients with BCR after RP, PET/CT detection rates are only 5–24% when the PSA level is < 1 ng/mL but rise to 67–100% when the PSA level is > 5 ng/mL. Despite its limitations, choline PET/CT may change medical management in 18–48% of patients with BCR after primary treatment [971-973].
Choline PET/CT should only be recommended in patients fit enough for curative loco-regional salvage treatment.
6.4.4.1.3. Fluoride PET/CT
18F-NaF PET/CT has a higher sensitivity than bone scan in detecting bone metastases [974]. However, 18F -NaF PET/CT is limited by a relative lack of specificity and by the fact that it does not assess soft-tissue metastases [975].
6.4.4.1.4. Fluciclovine PET/CT
18F-Fluciclovine PET/CT has been approved in the U.S. and Europe and it is therefore one of the PCa-specific radiotracers widely commercially available [975-978].
18F-Fluciclovine PET/CT has a slightly higher sensitivity than choline PET/CT in detecting the site of relapse in BCR [979]. In a multi-centre trial evaluating 596 patients with BCR in a mixed population, fluciclovine PET/CT showed an overall detection rate of 67.7%; lesions could be visualised either at local level (38.7%) or in pelvic LNs (32.6%) [980]. As for choline PET/CT, fluciclovine PET/CT sensitivity is dependent on the PSA level, with a sensitivity likely inferior to 50% at PSA level < 1 ng/mL.
In a prospective RCT evaluating the impact of 18F-fluciclovine PET/CT on SRT management decisions in patients with recurrence post-prostatectomy, in 28 of 79 (35.4%) patients overall radiotherapeutic management changed following 18F-fluciclovine PET/CT [981]. 18F-Fluciclovine PET/CT had a significantly higher positivity rate than conventional imaging (abdominopelvic CT or MRI plus bone scan) for whole body (79.7% vs. 13.9%, p < 0.001), prostate bed (69.6% vs. 5.1%, p < 0.001), and pelvic LNs (38.0% vs. 10.1%, p < 0.001) [981]. However, as yet, no data demonstrating that these changes translate into a survival benefit are available.
6.4.4.1.5. Prostate-specific membrane antigen based PET/CT
PSMA PET/CT has shown good potential in patients with BCR. The diagnostic performance of 18F-PSMA PET/CT in patients with BCR has been recently investigated by means of a systematic review and meta-analysis. The pooled sensitivity, specificity, and AUC values for 18F-PSMA PET/CT in the diagnosis of prostate recurrence and/or metastasis were 0.93 (0.89–0.95), 0.94 (0.85–0.98), and 0.96 (0,94–0.98), respectively the per-patient pooled sensitivity and specificity values were 0.92 (0.86–0.96) and 0.83 (0.41–0.97), respectively. The per-lesion pooled sensitivity and specificity values were identical, 0.91 (0.86–0.94) [982].
Reported predictors of 68Ga-PSMA PET in the recurrence setting were updated based on a high-volume series (Table 6.4.2) [867]. High sensitivity (75%) and specificity (99%) were observed on per-lesion analysis.
PSMA PET/CT seems substantially more sensitive than choline PET/CT, especially for PSA levels < 1 ng/mL [983,984]. In a study of 314 patients with BCR after treatment and a median PSA level of 0.83 ng/mL, 68Ga-PSMA PET/CT was positive in 197 patients (67%) [985]. In a phase III, prospective, multicentre, randomised study, comparing 18F-PSMA-1007 and 18FCholine PET/CT in PCa patients with biochemical recurrence, the overall correct detection rate (DR) was 84% (95% CI: 0.7967–0.8830) for PSMA and 69% (95% CI: 0.6191–0.7489) for choline. This yielded a significant proportion difference of 16% (P < 0.0001). Also, the DR for cutoff point PSA ≤ 1ng/ml was higher for PSMA compared to Choline (61.8% vs. 39.5%) [986].
A prospective multi-centre, multi-reader, open-label, phase II/III trial (OSPREY) evaluated the diagnostic performance of 18F-DCFPyL in patients with presumptive radiologic evidence of recurrent or metastatic PCa on conventional imaging [907]. Median sensitivity and median PPV were 95.8% (95% CI: 87.8%–99.0%) and 81.9% (95% CI: 73.7%–90.2%), respectively.
Another prospective study evaluated the diagnostic performance of 18F-DCFPyL in 208 men with BCR after RP or RT. The primary endpoint, the correct localisation rate was achieved, demonstrating positive findings on 18F-DCFPyL PET/CT in the setting of negative standard conventional imaging [987]. At present there are no conclusive data about comparison of such tracers [988].
A prospective, open label, cross-over study, the PYTHON trial, has compared the per-patient detection rates (DR) of 18F-DCFPyL versus 18F-fluoromethylcholine PET/CT, in biochemical recurrence (BCR) setting. A total of 201 high-risk PCa patients with first BCR after radical prostatectomy or radiation therapy have completed the study. The per-patient DR was significantly higher for 18F-DCFPyL compared to 18F-fluoromethylcholine PET/CT (58% (117/201 patients) vs. 40% (81/201 patients), p < 0.0001). DR increased with higher PSA values for both tracers (PSA ≤ 0.5 ng/ml: 26/74 (35%) vs. 22/74 (30%); PSA 0.5 to ≤ 1.0 ng/ml: 17/31 (55%) vs. 10/31 (32%); PSA 1.01 to < 2.0 ng/ml: 13/19 (68%) vs. 6/19 (32%);PSA > 2.0: 50/57 (88%) vs. 39/57 (68%) for 18FDCFPyL- and 18F-fluoromethylcholine -PET/CT, respectively) [989]. Comparable results were found in a phase III trial of 18F-PSMA-1007 versus 18F-Fluorocholine PET/CT for the localisation of prostate cancer biochemical recurrence. In this prospective, randomised, crossover multi-centre study, the overall correct detection rates were significantly higher for 18F-PSMA-1007 than for 18F-fluorocholine when undetermined findings were considered positive for malignancy (0.82 vs. 0.65; p < 0.0001) [990].
Table 6.4.2: PSMA-positivity separated by PSA level category [991]
| PSA (ng/mL) | 68Ga-PMSA PET positivity |
| < 0.2 | 33% (CI: 16–51) |
| 0.2–0.49 | 45% (CI: 39–52) |
| 0.5–0.99 | 59% (CI: 50–68) |
| 1.0–1.99 | 75% (CI: 66–84) |
| 2.0+ | 95% (CI: 92–97) |
PSA = prostate-specific antigen; 68Ga-PSMA PET = Gallium-68 prostate-specific membrane antigen positronemission tomography.
6.4.4.1.6. Whole-body and axial MRI
Whole body MRI has not been widely evaluated in BCR because of its limited value in the detection of early metastatic involvement in normal-sized LNs [455,486,992]. In a prospective series of 68 patients with BCR, the diagnostic performance of DW-MRI was significantly lower than that of 68Ga-PSMA PET/CT and 18NaF PET/CT for diagnosing bone metastases [993].
6.4.4.2. Assessment of local recurrences
6.4.4.2.1. Local recurrence after radical prostatectomy
Because the sensitivity of anastomotic biopsies is low, especially for PSA levels < 1 ng/mL [961], SRT is usually decided on the basis of BCR without histological proof of local recurrence.
Magnetic resonance imaging can detect local recurrences in the prostatic bed. The PSA threshold for MRI positivity seems between 0.3 and 0.5 ng/mL; PSA kinetics also influence the MRI positivity, even at low PSA values [994]. Two single-centre studies found that a negative MRI was an independent predictor of failure of SRT [995,996]. Conversely, a small (≤0.4 cc) relapse located at the vesico-urethral anastomosis is associated with excellent prognosis at salvage RT [997]. The Prostate Imaging for Recurrence Reporting (PI-RR) system has been recently launched to standardise MRI interpretation in the context of BCR after RP or RT [998]. Initial assessment suggests good reproducibility of the score [999].
Choline PET/CT is less sensitive for local relapse than MRI but detects more regional and distant metastases [1000].
The detection rates of 68Ga-PSMA PET/CT in patients with BCR after RP increase with the PSA level [1001]. PSMA PET/CT studies showed that a substantial part of recurrences after RP were located outside the prostatic fossa, even at low PSA levels [1002,1003]. Combining 68Ga-PSMA PET and MRI may improve the detection of local recurrences, as compared to 68Ga-PSMA PET/CT alone [1004-1006].
The EMPIRE-1, a single-centre, open-label, phase II/III RCT included 365 patients with detectable PSA after RP, but negative results on conventional imaging. They were randomised to RT directed by conventional imaging alone or to conventional imaging plus 18F-fluciclovine-PET/CT; patients with M1 disease in the PET/CT group (n = 4) were excluded Patients with cN1 were irradiated to the pelvic nodes, but without a boost to the metastases. After a median follow-up of 3.5 years, the PET/CT group was significantly associated with longer event-free survival (HR: 2.04, 95% CI: 1.06–3.93, p = 0.0327) [1007].
6.4.4.2.2. Local recurrence after radiation therapy
In patients with BCR after RT, biopsy status is a major predictor of outcome, provided the biopsies are obtained 18–24 months after initial treatment. Given the morbidity of local salvage options it is necessary to obtain histological proof of the local recurrence before treating the patient [961].
MRI has yielded excellent results in identifying local recurrence and can be used for biopsy targeting and guiding local salvage treatment [961,1008,1009], even if it slightly underestimates the volume of the local recurrence [1010]. Prostate-specific membrane antigen PET/CT can also detect local recurrences after RT [991] and concordance between PSMA PET/CT and MRI is highly suggestive of cancer recurrence [1011].
6.4.4.3. Summary of evidence of imaging in case of biochemical recurrence
In patients with BCR imaging can detect both local recurrences and distant metastases, however, the sensitivity of detection depends on the PSA level. After RP, PSMA PET/CT is the imaging modality with the highest sensitivity at low PSA levels (< 0.5 ng/mL) and may help distinguishing patients with recurrences confined to the prostatic fossa from those with distant metastases which may impact the design and use of post-RP SRT. After RT, MRI has shown excellent results at detecting local recurrences and guiding prostate biopsy. Given the substantial morbidity of post-RT local salvage treatments, distant metastases must be ruled out in patients with local recurrences and who are fit for these salvage therapies. Choline-, fluciclovine- or PSMA-PET/CT can be used to detect metastases in these patients but for this indication PSMA PET/CT seems the most sensitive technique.
6.4.4.4. Recommendations for imaging in patients with biochemical recurrence
| Recommendations | Strength rating |
| Prostate-specific antigen (PSA) recurrence after radical prostatectomy | |
| Perform prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) if the PSA level is > 0.2 ng/mL and if the results will influence subsequent treatment decisions (EAU BCR risk groups). | Weak |
| In PSMA PET/CT is not available, and the PSA level is ≥ 1 ng/mL, perform fluciclovine PET/CT or choline PET/CT imaging if the results will influence subsequent treatment decisions. | Weak |
| PSA recurrence after radiotherapy | |
| Perform prostate magnetic resonance imaging to localise abnormal areas and guide biopsies in patients fit for local salvage therapy. | Weak |
| Perform PSMA PET/CT (if available) or fluciclovine PET/CT or choline PET/CT in patients fit for curative salvage treatment. | Strong |
6.4.5. Treatment of PSA-only recurrences
The timing and treatment modality for PSA-only recurrences after RP or RT remain a matter of controversy based on the limited evidence.
6.4.5.1. Treatment of PSA-only recurrences after radical prostatectomy
6.4.5.1.1. Salvage radiotherapy for PSA-only recurrence after radical prostatectomy (cTxcN0M0, without PET/CT)
Early SRT provides the possibility of cure for patients with an increasing PSA after RP. Boorjian et al., reported a 75% reduced risk of systemic progression with SRT when comparing 856 SRT patients with 1,801 non-SRT patients [1012]. The RAVES and RADICAL trials assessing SRT in post-RP patients with PSA levels exceeding 0.1–0.2 ng/mL showed 5-year freedom from BCR and BCR-free survival rates of 88% [999,1013]. Tilki et al. demonstrated the results of a matched pair analysis of 1832 patients with BCR, 32.9% (n = 603) received SRT without ADT, 1229 (67,1%) had a observational strategy. The median follow-up was 95.9 months. Median total SRT dose was 70.2 Gy. After 1:1 propensity score matching, at fiveteen years after RP, MFS and OS rates were 84.3 versus 76.9% (p < 0.001) and 85.3 versus 74.4% (p = 0.04) for SRT and noRT, respectively [1014].
The PSA level at BCR was shown to be prognostic [1012]. More than 60% of patients who are treated before the PSA level rises to > 0.5 ng/mL will achieve an undetectable PSA level [1015-1017], corresponding to a ~80% chance of being progression-free five years later [1018]. A retrospective analysis of 635 patients who were followed after RP and experienced BCR and/or local recurrence and either received no salvage treatment (n = 397) or SRT alone (n = 160) within two years of BCR showed that SRT was associated with a 3-fold increase in PCa-specific survival relative to those who received no salvage treatment (p < 0.001). Salvage RT has been shown to be effective mainly in patients with a short PSA-DT [1019].
In a retrospective multi-centre study including 25,551 patients with at most one high-risk factor after RP (ISUP grade group 4-5 or pT3/4), initiating sRT above a PSA level of 0.25 ng/mL was associated with increased ACM-risk. After a median follow-up of six years, patients who received sRT at a PSA level >0.25 ng/mL had a significantly higher ACM-risk (AHR, 1.49; 95% CI, 1.11 to 2.00; P =.008) compared with men who received sRT when the PSA was ≤0.25 mg/mL [1020]. For an overview of SRT see Table 6.4.3.
Although biochemical progression is now widely accepted as a surrogate marker of PCa recurrence; metastatic disease, disease-specific and OS are more meaningful endpoints to support clinical decision-making. A SR and meta-analysis on the impact of BCR after RP reports SRT to be favourable for OS and PCSM. In particular SRT should be initiated in patients with rapid PSA kinetics after RP and with a PSA cut-off of 0.4 ng/mL [933]. An international multi-institutional analysis of pooled data from RCTs has suggested that MFS is the most valid surrogate endpoint with respect to impact on OS [1021,1022]. Table 6.4.4 summarises results of recent studies on clinical endpoints after SRT.
The EAU BCR definitions have been externally validated and may be helpful for individualised treatment decisions [933,959]. Despite the indication for salvage RT, a ‘wait and see‘ strategy remains an option for the EAU BCR ‘Low-Risk’ group [933,959].
6.4.5.1.2. Salvage radiotherapy combined with androgen deprivation therapy (cTxcN0, without PET/CT)
Data from RTOG 9601 suggest both CSS and OS benefit when adding two years of bicalutamide (150 mg o.d.) to SRT [1023]. According to GETUG-AFU 16 also 6-months treatment with a LHRH-analogue can significantly improve 10-year BCR, biochemical PFS and, modestly, MFS. However, SRT combined with either goserelin or placebo showed similar DSS and OS rates [1024].
In addition, Pollack et al., reported on the results of a randomised 3-arm phase III trial (NRG Oncology/RTOG 0534 SPPORT) adding six months treatment with a LHRH-analogue to SRT of the prostate bed (PBRT) (group 2) compared with PBRT alone (group 1) or the former combination with PBRT-RT and pelvic LN RT (PLNRT) (group 3) [1025]. The primary endpoint was freedom from progression (FFP) after five years. However, using the phoenix-definition of biochemical progression (nadir + 2 ng/mL used for definitive RT), and not the criterion of nadir + 0.2, as is used commonly (but without clear evidence) will have resulted in a later diagnosis of progression in the SPPORT trial.
With a median follow-up of 8.2 years of the surviving patients FFP increased significantly for group 3 (87.4%) compared with group 2 (81.3%) (p = 0.0027) and group 1 (70.9%) (p < 0.0001) [1025]. The difference between group 2 and group 1 was also significant (p < 0.0001). Distant metastasis incidence rates (secondary endpoint) were lowest in group 3 (including RT of the pelvic lymphatics) and were significantly lower only compared with group 1 (PBRT only, HR: 0.52) similar to the rate of PCa deaths (HR: 0.51). No significant difference was seen for OS. There was a significantly higher risk of both acute- and late side effects in group 3. Therefore, the role of additional PLNRT remains unclear and should be further proven in RCTs including PSMA PET-CT [1026].
RADICALS HD investigated the role of RT without ADT (n = 737) versus RT plus 6 months ADT (n = 747) and RT plus 6 months ADT (n = 761) versus RT plus 24 months long term ADT (n = 762) in both the salvage and adjuvant settings [1027-1029]. The design of RADICALS HD was complex and included components of the RADICALS RT trial together with the RADICALS HD component. RADICALS RT was a phase III comparison of ART versus observation and early SRT and has been published previously [901] (see table 6.3.5.2).
RADICALS HD included men after prostatectomy (indications for ART or early SRT), median pre SRT-PSA was 0.2 ng/ml with conventional staging imaging (M0) without PET-CT. Due to the complex design some patients were enrolled in a three-way randomisation (including patients from RADICALS RT, n = 492) and in a two-way randomisation (SRT + 6 months- or 24 months ADT, n = 1,197). The randomisation was influenced by physician preference. For this reason, more patients had high risk factors in the short term ADT- versus long term ADT-study (ISUP >3: 29% versus 11% and for > pT3B-tumours: 31% vs. 17%) compared with the no ADT- and the short term ADT-study [1030].
With a median FU of nine years the ten-year MFS (primary endpoint, inclusion of deaths from PCa only) for no ADT vs. short term ADT showed no significant difference for both arms (88.1% vs. 89.9%, p>0.05) but for “Clinical progression free survival” (68.3% versus 79.4%, p<0.0001 with some evidence of non proportional hazards) and “10-year freedom from non protocol ADT” (73.3% vs. 82.3%, p<0.0001 but with clear evidence of non-proportional hazards). Max. GU-Tox grade 3 was 16% (no ADT) versus 13% (short term ADT) (p>0.05). With a median FU of 8.9 years the 10-year MFS (primary endpoint) in the second radomisation showed a moderate significant difference (78.1% vs. 71.9%) in favour of the long term ADT arm compared with the short term ADT arm (p=0.029, HR 0.773). Comparable significant differences were seen for “Clinical progression free survival” and for “10-year freedom from non-protocol ADT”. Max. GU-Tox grade 3 was 14% (short term ADT) vs. 20% (long term ADT).
The authors concluded, that the findings for short term ADT versus no ADT do not support the use of ADT in this patient population. For the comparison of long term ADT versus short term ADT the conclusion was that “individuals who can accept the additional duration of adverse effects, long-course ADT should be offered” with SRT.
Table 6.4.5 provides an overview of these five RCTs. One of these RCTs reports improved OS (RTOG 96-01), another (GETUG-AFU 16) improved moderately MFS (7%) at 10 years. The SPPORT trial improved FFP for all three arms and the distant metastasis rate only in the comparison of PBRT+ RT of the pelvic lymphatics + 6 month ADT what makes the interpretation difficult. The two arm comparision (SRT versus SRT + 6 months ADT) of RADICALS HD did not improve MFS after 10 years, this is in contrast to the results of the GETUG-AFU 16 trial. Only the comparison of SRT + 6 months ADT versus SRT+ 24 months ADT of RADICALS HD improved moderately 10 year MFS (6.2%). This improvement came on the cost of increased side effects of the additional 18 months ADT including a double rate of patients with testosteron-supression after 10 years compared with 6 months of ADT [1031].
Due to methodological discrepancies and also related to follow-up and risk, it is, as yet, not evident which patients should receive ADT, which type of ADT, and for how long. Men at high risk of further progression (e.g., with a PSA ≥ 0.7 ng/mL and GS ≥ 8) may benefit from SRT combined with two years of ADT; for those at lower risk (e.g., PSA < 0.7 ng/mL and GS = 8) SRT combined with six months of ADT may be sufficient [1023]. Men with a low-risk profile (PSA < 0.5 ng/mL and GS < 8) and a PSA level <0.5 ng/ml may receive SRT alone. In a unplanned subgroup-analysis [1032] (RTOG 96-01) of men with a PSA of 0.61 to 1.5 (n = 253) there was an OS benefit associated with antiandrogen assignment (HR: 0.61, 95% CI: 0.39–0.94) [1032]. In those receiving early SRT (PSA <0.6 ng/mL, n = 389), there was no improvement in OS (HR: 1.16, 95% CI: 0.79–1.70), with increased other-cause mortality (sub-distribution HR: 1.94, 95% CI: 1.17–3.20, p = 0.01) and increased odds of late grades 3–5 cardiac and neurologic toxic side effects (OR: 3.57; 95% CI: 1.09–15.97, p = 0.05). These results suggest that pre-SRT PSA level may be a prognostic biomarker for outcomes of anti-androgen treatment with SRT. A SR addressing the benefit from combining HT with SRT suggested risk stratification of patients based on the pre-SRT PSA (< 0.5, 0.6–1, > 1 ng/mL), margin status and ISUP grade as a framework to individualise treatment [1033]. In addition, potential risk factors that should be considered are (short) PSA-doubling time and pT3b-4-tumours [1027,1028,1030].
In conclusion regarding the “weak” recommendation “offer hormonal therapy in addition to SRT to men with BCR we have different results of three RCT’s for additional short term ADT (6 months) to SRT. One showed an increase of MFS [1024], the second and third one did not [1025,1028]. Of two RCT’s with long term ADT in addition to SRT one RCT showed a significant better OS [1023], the second one did not [1028] but this one showed a moderate increase in MFS with the cost of a higher rate of severe side effects. Additionally in RADICALS HD no subgroup analysis of risk factors was performed. To establish more precise recommendations 10-year results of the other RCT’s and the meta-analysis have to be awaited.
6.4.5.1.2.1. Target volume, dose, toxicity
There have been various attempts to define common outlines for ‘clinical target volumes‘ for pN0 PCa [1034,1035] and for organs at risk of normal tissue complications [1034]. However, given the variations of techniques and dose-constraints, a satisfactory consensus has not yet been achieved. A benefit in biochemical PFS but not MFS has been reported in patients receiving whole pelvis SRT (± ADT) but the advantages must be weighed against possible side effects [1026]. This is supported by data from the SPPORTTrial (NRG Oncology/RTOG 0534 SPPORT) but it remains controversial [1025].
The optimal SRT dose has not been well defined. It should be at least 64 Gy to the prostatic fossa (± the base of the SVs, depending on the pathological stage after RP) [904,1036]. In a SR, the pre- SRT PSA level and SRT dose both correlated with BCR, showing that relapse-free survival decreased by 2.4% per 0.1 ng/mL PSA and improved by 2.6% per Gy, suggesting that the treatment dose above 70 Gy should be administered at the lowest possible PSA level [1037]. The combination of pT stage, margin status and ISUP grade group and the PSA at SRT seems to define the risk of biochemical progression, metastasis and overall mortality [894,1038]. In a study on 894 node-negative PCa patients, doses ranging from 64 to > 74 Gy were assigned to twelve risk groups defined by their pre-SRT PSA classes < 0.1, 0.1–0.2, 0.2–0.4, and > 0.4 ng/mL and ISUP grade group < 1 vs. 2/3 vs.
> 4 [1039]. The updated Stephenson nomograms incorporate the SRT and ADT doses as predictive factors for biochemical failure and distant metastasis [1040].
Two RCT’s were published (Table 6.4.6). Intensity-modulated radiation therapy plus IGRT was used in 57% of the patients in the SAKK-trial [1041] and in all patients of a Chinese trial [1042]. No patient had a PSMA PET/CT before randomisation. The primary endpoint in both trials was ‘freedom from biochemical progression’, which was not significantly improved with higher doses. However, in the Chinese trial a subgroup analysis showed a significant improvement of this endpoint for patients with Gleason 8-10 tumours (66.5% vs. 30.2%, p = 0.012) and for multiple positive surgical margins (82.5% vs. 57.5%, p=0.037) [1042]. In this trial, patients were treated with ART or SRT and the number of patients was relatively small (n = 144). At this time it seems difficult to draw final conclusions about the optimal total RT-dose and longer follow-up should be awaited but subgroups of high-risk patients might profit from higher total doses.
Salvage RT is associated with toxicity. In one report on 464 SRT patients receiving median 66.6 (max. 72) Gy, acute grade 2 toxicity was recorded in 4.7% for both the GI and GU tract. Two men had late grade 3 reactions of the GI tract, but overall, severe GU tract toxicity was not observed. Late grade 2 complications occurred in 4.7% (GI tract) and 4.1% (GU tract), respectively, and 4.5% of the patients developed moderate urethral stricture [1043].
In a RCT on dose escalation for SRT (n = 350), acute grade 2 and 3 GU toxicity was observed in 13.0% and 0.6%, respectively, with 64 Gy and in 16.6% and 1.7%, respectively, with 70 Gy. Gastro-intestinal tract grades 2 and 3 toxicity occurred in 16.0% and 0.6%, respectively, with 64 Gy, and in 15.4% and 2.3%, respectively, with 70 Gy [1044,1045]. Late grade 2 and 3 GI toxicity was significantly increased with higher doses but without significant differences in QoL. In this study, however, the rectal wall dose constraints were rather permissive and in 44% of the patients outdated 3-D-techniques were used [1041].
With dose escalation over 72 Gy and/or up to a median of 76 Gy, the rate of severe side effects, especially GU symptoms, clearly increases, even with newer planning and treatment techniques [1046,1047]. In particular, when compared with 3D-CRT, IMRT was associated with a reduction in grade 2 GI toxicity from 10.2 to 1.9% (p = 0.02) but no effect on the relatively high level of GU toxicity was shown (5-year, 3D-CRT 15.8% vs. IMRT 16.8%) [1046]. However, in a RCT comparing 66 Gy and 72 Gy with all patients having IMRT plus IGRT (n = 144), no significant differences for GI and GU-toxicity was demonstrated [1048]. After a median salvage IMRT dose of 76 Gy however, the 5-year risk of grade 2–3 toxicity rose to 22% for GU and 8% for GI symptoms, respectively [1047]. Doses of at least 64 Gy and up to 72 Gy in patients without PET/CT can be recommended [1043,1044].
Table 6.4.3: Selected studies of post-prostatectomy salvage radiotherapy, stratified by pre-salvage radiotherapy PSA level* (cTxcN0M0, without PET/CT)
| Selected studies of post-prostatectomy salvage radiotherapy | ||||||
| Study | n | Median FU (mo) | pre-SRT PSA (ng/mL) median | RT dose ADT | bNED/PFS (year) | 5-yr. results |
| Bartkowiak, et al. 2018 [1043] | 464 | 71 | 0.31 | 66.6 Gy | 54% (5.9) | 73% vs. 56%; PSA < 0.2 vs. ≥ 0.2 ng/mLp < 0.0001 |
| Stish, et al. 2016 [1015] | 1,106 | 107 | 0.6 | 68 Gy 16% ADT | 50% (5) 36% (10) | 44% vs. 58%; PSA < 0.5 vs. > 0.5 ng/mL |
| Tendulkar, et al. 2016 [1040] | 2,460 | 60 | 0.5 | 66 Gy 16% ADT | 56% (5) | Pre-SRT PSA 71% 0.01–0.2 ng/mL 63% 0.21–0.5 ng/mL 54% 0.51–1.0 ng/mL 43% 1.01–2.0 ng/mL 37% > 2.0 ng/mL |
| Tilki et al. 2023 [1020] | 25,551 SRT at: PSA < 0.25 n=1,556 PSA > 0.25 n=1,677 No RT: n=21,645 | 72 | Not given | Med. 68.4 Gy SRT+ADT:1489 ART:N= 673 ADT: 208
| Not given | ACM (six years): HR 1.49 of higher risk when SRT at start was > 0.25 (p=0.008) |
*Androgen deprivation therapy can influence the outcome ‘biochemically no evidence of disease (bNED)’ or ‘progression-free survival’. To facilitate comparisons, 5-year bNED/PFS read-outs from Kaplan-Meier plots are included.
ADT = androgen deprivation therapy; bNED = biochemically no evidence of disease; FU = follow up; mo = months; n = number of patients; PFS = progression-free survival; PSA = prostate-specific antigen; SRT = salvage radiotherapy; yr = year.
Table 6.4.4: Selected studies reporting clinical endpoints after SRT (cTxcN0M0, without PET/CT)(the majority of included patients did not receive ADT)
| Selected studies reporting clinical endpoints after SRT | ||||
| Study | n | Median FU (mo) | Regimen | Outcome |
| Bartkowiak, et al. 2018 [1043] | 464 | 71 | 66.6 (59.4-72) Gy no ADT | 5.9 yr. OS post-SRT PSA < 0.1 ng/mL 98% post-SRT PSA > 0.1 ng/mL 92% p = 0.005 |
| Jackson, et al. 2014 [1049] | 448 | 64 | 68.4 Gy no ADT | 5 yr. DM post-SRT PSA < 0.1 ng/mL 5% post-SRT PSA > 0.1 ng/mL 29% p < 0.0001 5 yr. DSM post-SRT PSA < 0.1 ng/mL 2% post-SRT PSA > 0.1 ng/mL 7% p < 0.0001 OS post-SRT PSA < 0.1 ng/mL 97% post-SRT PSA > 0.1 ng/mL 90% p < 0.0001 |
| Stish, et al. 2016 [1015] | 1,106 | 107 | 68 (64.8-70.2) Gy 39% 2D treatment planning incl. 16% ADT | 5 and 8.9 yr. DM SRT: PSA < 0.5 ng/mL 7% and 12% SRT: PSA > 0.5 ng/mL 14% and 23% p < 0.001 5 and 8.9 yr. DSM SRT: PSA < 0.5 ng/mL < 1% and 6% SRT: PSA > 0.5 ng/mL 5% and 10% p = 0.02 5 and 8.9 yr. OS SRT: PSA < 0.5 ng/mL 94% and 86% SRT: PSA > 0.5 ng/mL 91% and 78% p = 0.14 |
| Tendulkar, et al. 2016 [1040] | 2,460 | 60 | 66 (64.8-68.4) Gy incl. 16% ADT | 10-yr. DM (19% all patients) Pre-SRT PSA 9% 0.01–0.2 ng/mL 15% 0.21–0.5 ng/mL 19% 0.51–1.0 ng/mL 20% 1.01–2.0 ng/mL 37% > 2.0 ng/mL p < 0.001 |
ADT = androgen deprivation therapy; DM = distant metastasis; DSM = disease specific mortality;FU = follow up; mo. = month; n = number of patients; OS = overall survival; PSA = prostate specific antigen; SRT = salvage radiotherapy.
Table 6.4.5: Randomised controlled trials comparing salvage radiotherapy combined with androgen deprivation therapy vs. salvage radiotherapy alone
| Study | |||||
| Study | n | Risk groups | Median FU (mo) | Regimen | Outcome |
| GETUG-AFU 16 2019 [1024] | 369 SRT + ADT 374 RT | ISUP GG ≤ 2/3 89% SUP GG ≥ 4 11% cN0 | 112 | 66 Gy PBRT+ 6 mo. LHRH analogue 66 Gy PBRT | 10-yr. PFS: RT + ADT, 64% PFS: RT, 49% p < 0.0001 MFS: RT + ADT, 75% MFS: RT, 69% p = 0.034 |
RTOG 9601 2017 [1023] | 384 SRT + ADT 376 SRT | pT2 R1, pT3 cN0 | 156 | 64.8 Gy PBRT + bicalutamide 24 mo. 64.8 Gy PBRT + placebo | 12-yr. cumulative DM RT + ADT: 14% RT + placebo: 23% p = 0.005 OS RT + ADT: 76% RT + placebo: 71% p = 0.04 DSM RT + ADT: 5.8% RT + placebo: 13.4% p < 0.001 |
| NRG Oncology/RTOG 0534 SPPORT [1025] | 564 SRT 578 SRT + ADT 574 SRT + PBRT + ADT | pT2 or pT3 ISUP GG <5 Pre SRT PSA: 0.1-2.0 | survivors: 8.2 years | 64.8–0.2 Gy PBRT 64.8–70.2 Gy PBRT 6 mo. LHRH analogue 64.8–70.2 Gy PBRT + 45 Gy PLNRT 6 mo. LHRH analogue | 5-yr. FFP (primary endpoint) 70.9% Group 1 81.3% Group 2 87.4% Group 3 Comparisons : G 3 vs. G 1: p < 0.0001 G 2 vs. G 1: p < 0.0001 G 3 vs. G 2: p < 0.0027 |
RADICALS HD 0 vs. 6 mo. ADT [1027] | 737 SRT 747 SRT+ADT | ISUP >7 (11%) ≥ pT3b (17%) R1 (62%) PSA: < 0.3 (61%) ≥ 0.5 (19%) R1 (62%) N1 (3%) | 108 | 52.5 Gy, 20 Fx PBRT (29%) 66 Gy, 33 Fx PBRT (69%) LHRH analogue (83%) | 10-yr. MFS: SRT: 79.2% SRT+ADT: 80.4% p= 0.71; HR: 0.89 CPFS: SRT: 68.3% SRT+ADT: 79.4% p = 0.071, HR:0.54 Max.GU-Tox G 3: SRT: 16% SRT+ADT: 13% p>0.05 |
RADICALS HD 6 versus 24 months ADT [1028] | 761 6 mo. ADT
762 24 mo. ADT | ISUP > 7 (29%) ≥ pT3b (31%) Med. Pre SRT PSA: 0.23 R1 (63%) N1 (8%) | 107 | 52.5 Gy 20 Fx PBRT (19%) 66 Gy, 33 Fx PBRT (79%) LHRH analogue (84%) | 10 year. MFS: SRT+6 mo.: 71.9% SRT+24 mo.: 78.1% p = 0.029, HR: 0.77 Max.GU-Tox G3: SRT+6 mo.: 14% SRT+24 mo.:20% p = 0.025 |
ADT = androgen deprivation therapy; CPFS= clinical progression free survival; DM = distant metastasis; DSM = disease specific mortality; PFS = progression free survival; FFP = Freedom From Progression; FU = follow-up; LHRH = luteinising hormone-releasing hormone; MFS = metastasis-free survival; OS = overall survival; PFS = progression-free survival; mo = months; n = number of patients; RT = radiotherapy; yr = year, PBRT = prostate bed radiotherapy; PLNRT = pelvic lymph node radiotherapy.
Table 6.4.6: Randomised trials investigating dose escalation for SRT without ADT and without PET-CT
| Randomised trials investigating dose escalation for SRT without ADT and without PET-CT | ||||||
| Trial | n | PCa condition | Radiotherapy Dose | Follow-up (median) | Outcome | Results |
| SAKK 09/10 trial, 2021 [904] | 350 | pT2a-3b R0 – R1 pN0 or cN0 PSA post op undetectable (< 0.1 ng/mL) or persistent (> 0.1 ng/mL < 0.4 ng/mL) | 64 Gy vs.70 Gy
No ADT allowed
VMAT+ IGRT: 57% 3-D planning: 43% | 6.2 yr. | Primary endpoint: FFBP | 6 yr. FFBP: 62% vs. 61% OS: no difference Late side effects: GI grade 2: 7.3% vs. 20% GI grade 3: 4.2% vs. 2.3% p for ≥ grade 2/3: 0.009 |
Phase-III-Trial Qi X, et al., 2024 [1042]
| 144 ART: 33% SRT: 67% | pT2-4 R0-R1 pN0 or cN0 Med. PSA pre-RT: 0.2 ng/mL | 66 Gy vs.72 Gy All patients VMAT+ IGRT No ADT allowed High risk (pT3-4, GS: 8-10, PSA >20 ng/mL): whole pelvis RT: 126 (87.5%) | 89.5 mo. | Primary endpoint: FFBP | 7 yr. FFBP: 70.3% vs. 61.2% (p > 0.05) High risk (GS: 8–10): 66.5% vs. 30.2% p < 0.012 HR: 0.73 Multiple SR+: 82.5% vs. 57.5% p=0.037 HR: 0.36 Late side effects: GI + GU grade 2 p > 0.05 No grade 3 |
ADT = androgen deprivation therapy; ART = adjuvant radiotherapy; FFBP = freedom from biochemical failure; GI = gastro-intestinal; GU = genito-urinary; Gy = Gray; IGRT = image guided radiotherapy; mo = month; n = number of patients; PSA = prostate-specific antigen; RT = radiotherapy; SRT = y = year; vs. = versus; VMAT = volumetric arc radiation therapy.
6.4.5.1.2.2. Salvage radiotherapy with or without ADT (cTx cN0/1) with PET/CT
In a prospective multi-centre study of 323 patients with BCR, PSMA PET/CT changed the management intent in 62% of patients as compared to conventional staging. This was due to a significant reduction in the number of men in whom the site of disease recurrence was unknown (77% vs. 19%, p < 0.001) and a significant increase in the number of men with metastatic disease (11% vs. 57%) [1050]. A prospective study in a subgroup of 119 BCR patients with low PSA (< 0.5 ng/mL) reported a change in the intended treatment in 30.2% of patients [1003]; however, no data exist on the impact on final outcome.
Another prospective study in 272 patients with early biochemical recurrent PCa after RP showed that 68Ga-PSMA PET/CT may tailor further therapy decisions (e.g., local vs. systemic treatment) at low PSA values (0.2–1 ng/mL) [1051].
A multi-centre retrospective study evaluated patients who underwent SRT for BCR after RP, without any signs of distant metastatic disease on PET/CT. After case-control matching, two cohorts (n = 108 patients each), with and without PSMA PET/CT prior to SRT were analysed. In the cohort without PSMA PET/CT, 23 patients (21%) had BCR at one year after SRT vs. nine patients (8%) who underwent restaging with PSMA PET/C prior to SRT (p = 0.007). PSMA-PET/CT was found to be associated with an improved oncological outcome in patients with BCR after RP, receiving SRT to the prostatic fossa [1052]. It is worth mentioning that in this study the median biologically effective radiation dose administered in the PSMA-cohort was significantly higher than in the historical cohort (70 Gy vs. 66 Gy, respectively, p < 0.001).
A single-centre open-label, phase II/III RCT (EMPIRE-1) evaluated the role of 18F-fluciclovine-PET/CT compared with conventional imaging for SRT. Three hundred and sixty five patients with detectable PSA after RP but negative results on conventional imaging, were randomised to RT directed by conventional imaging alone or to conventional imaging plus PET/CT; patients with M1 disease in the PET/CT group (n = 4) were excluded. Patients with cN1 were irradiated to the pelvic lymphatics but without a boost to the metastasis. Median follow 103 up was 3.5 years. In adjusted analyses, the study group was significantly associated with an improvement of the event-free survival (HR: 2.04, 95% CI: 1.06–3.93, p = 0.0327) [1007].
6.4.5.1.2.3. Nodal-directed therapy for rcN1 (with PET/CT)
Radiolabelled PSMA PET/CT is increasingly used as a diagnostic tool to assess metastatic disease burden in patients with BCR following prior definitive therapy. A review including 30 studies and 4,476 patients showed overall estimates of positivity in a restaging setting of 38% in pelvic LNs and 13% in extra-pelvic LN metastases [991]. The percentage positivity of PSMA PET/CT was proven to increase with higher PSA values [991]. Results of this review demonstrated a high sensitivity and specificity of 68Ga-PSMA in advanced PCa, with a per-lesion-analysed sensitivity and specificity of 75% and 99%, respectively.
A large retrospective international study included patients with LN-recurrent PCa (cN1 and M1a) and PSA progression following multi-modality treatment (surgery and post-operative RT) [1053]. The aim of the study was to compare SOC with nodal metastasis-directed therapy (MDT). The nodal MDT-group showed significantly better CSS than the SOC control group (5-year survival 98.6% vs. 95.7%, p < 0.01, respectively) [1053].
Another retrospective study compared stereotactic body radiation therapy (SABR) with elective nodal irradiation (ENRT) in nodal oligo-recurrent PCa (n = 506 patients, 365 of which with N1 pelvic recurrence). With a median follow-up of 36 months, ENRT (n = 197) was associated with a significant reduction of nodal recurrences (p < 0.001), compared with SABR (n=309) of 2% vs. 18%, respectively. In a a multi-variable analysis, patients with one LN at recurrence had longer adjusted MFS after ENRT (HR: 0.50, 95% CI: 0.30–0.85, p = 0.009). The tendency to relapse was higher for pelvic- than extra-pelvic nodes (p < 0.001) [1054]. For patients presenting with two or more (extra)pelvic LNs, adjusted MFS was not significantly different (HR: 0.92, 95% CI: 0.54– 1.59, p = 0.8). In these situations, SABR should be used in highly selected patients in prospective cohorts or clinical trials only, before any recommendations can be made.
Long term outcomes have been reported from a prospective single arm study with extended-nodal radiotherapy (ENRT) and (11C)-choline PET-CT guided simultaneous integrated boost to positive lymph nodes in 60 patients [1055] 34 (56.7%) had a pelvic recurrence only. Median PSA relapse was 2.3 ng/ml and med. number of positive LN was 2. ADT was prescribed for 48/60 pts., median duration was 30.7 months, with 15/60 pts. had a castration-resistent PCa at diagnosis metastasis. The distant metastasis free-survival at ten years of the entire group was 45.2% [1055].
There is only one prospective Phase-II-trial (GETUG P07-OLIGOPELVIS) investigating the clinical outcome of IMRT+ADT in 67 patients with oligorecurrent (<=5) pelvic node relapses in fluorocholine positron-emission tomography CT-imaging [1056]. However, 61% of the patients had one positive node only. Median FU was 6.1 years. The 5-years PFS, bNED and ADT-free survival was 39%, 31% and 64% after elective RT of the pelvic lymphatics and 6 months ADT (LHRH agonist and antagonist). G 2+ 5-years GI and GU tox were 4% and 4%. The major site of relapse was para-aortic lymph nodes [1056].
In these situations, ENRT or SABR should be used in highly selected patients in prospective cohorts or clinical trials only, before any recommendations can be made. The optimal duration of ADT is uncertain and durations > 6 months are likely to be more effective. For MDT in M1 patients see section 6.6.7.
6.4.5.1.3. Salvage lymph node dissection
The surgical management of recurrent nodal metastases in the pelvis has been the topic of several retrospective analyses [1057-1059] and a SR [1060]. The reported 5-year BCR-free survival rates ranged from 6% to 31%. Five-year OS was approximately 84% [1060]. Biochemical recurrence rates were found to be dependent on PSA at salvage surgery and location and number of positive nodes [1061]. Addition of RT to the lymphatic template after salvage LN dissection may improve the BCR rate [1062]. In a multi-centre retrospective study long-term outcomes of 189 patients who underwent salvage LN dissection were reported to be worse than previously described in studies with shorter follow-up [1063]. Biochemical recurrence (BCR)-free survival at ten years was 11%. Patients with a PSA response after salvage LN dissection and patients receiving ADT within six months from salvage LN dissection had a lower risk of death from PCa [1063]. The majority of the patients (81%) had received a choline PET and median PSA at salvage LN dissection was 2.5 ng/mL. In a cohort study including patients treated with salvage LN dissection via PSMA--radioguided surgery (PSMA-RGS), 2-year BCR-free survival rate was 32% [1064]. In multi-variable analyses, higher pre-operative PSA, higher number of PSMA-avid lesions, multiple (pelvic plus retroperitoneal), and retroperitoneal localisation of lesions at pre-operative imaging were independent predictors of BCR after PSMA-RGS. High-level evidence for the oncological value of salvage LN dissection (including adjuvant RT of the LNs) is still lacking [1060].
6.4.5.2. Management of PSA failures after radiation therapy
Therapeutic options in these patients are ADT or salvage local procedures, as well as a ‘wait and see’ approach, based on EAU BCR risk categories at relapse. A SR and meta-analysis included studies comparing the efficacy and toxicity of salvage RP, salvage HIFU, salvage cryotherapy, SBRT, salvage LDR BT, and salvage HDR BT in the management of locally recurrent PCa after primary radical EBRT [1065]. The outcomes were BCR-free survival at two and five years. No significant differences with regards to recurrence-free survival (RFS) between these modalities was found. Five-year RFS ranged from 50% after cryotherapy to 60% after HDR BT and SBRT. The authors reported that severe GU toxicity exceeded 21% for whole-gland HIFU and RP, whereas it ranged from 4.2% to 8.1% with re-irradiation. Differences in severe GI toxicity also appeared to favour re-irradiation, particularly HDR BT [1065]. Due to the methodological limitations of this review (the majority of the included studies were uncontrolled single-arm case series and there was considerable heterogeneity in the definitions of core outcomes) the available evidence for these treatment options is of low quality and strong recommendations regarding the choice of any of these techniques cannot be made. The following is an overview of the most important findings for each of these techniques. Salvage cryo-therapy and focal HIFU are discussed in section 6.4.5.2.2
6.4.5.2.1. Salvage radical prostatectomy
Salvage RP after RT is associated with a higher likelihood of AEs compared to primary surgery because of the risk of fibrosis and poor wound healing due to radiation [1066].
6.4.5.2.1.1. Oncological outcomes
In a SR of the literature, Saouli et al., showed using data from 3836 patients in 55 studies across median follow-up ranging 4.6 – 94 months that SRP provided five-year BCR occurrence 48-59%, cancer-specific survival 13.4-98% and OS 62-100% [1067]. These figures are similar to those reported by Chade et al., in 2011, with 5-year and 10-year BCR-free survival estimates ranging from 47–82% and from 28–53%, respectively. The 10-year CSS and OS rates ranged from 70–83% and from 54–89%, respectively. The pre-SRP PSA value and initial prostate biopsy ISUP grade group were the strongest predictors of the presence of organ-confined disease, progression, and CSS [1068]. In a multi-centre analysis including 414 patients, 5-year BCR-free survival, CSS and OS were 56.7%, 97.7% and 92.1%, respectively [1069]. Pathological T stage ≥ T3b (OR: 2.348) and GS (up to OR: 7.183 for GS > 8) were independent predictors for BCR. Appropriate risk-stratification according to EAU Guidelines Biochemical Recurrence criteria may better select for SRP, with higher metastasis-free (90% vs 76%, p < 0.01) and OS
(89% vs 84%, p = 0.01) for low versus high EAU risk [1070,1071].
Lymphadenectomy was performed in most cases (79%), with 20.5% of patients staged N+ at final pathology [1067]. Detailed analysis of a multi-institutional series of 853 SRP patients reported that 87% underwent lymphadenectomy, 21% were pN1 and these patients suffered worse overall and cancer-specific survival [1072]. Like in primary surgery, patients with persistent PSA after SRP (42%) had worse BCR-free (6.6 vs 59%), metastasis-free (71 vs. 88%) and OS (77 vs. 94%) after median follow-up of 84 months according to a retrospective, multi-institutional series of 580 patients [1073]. Persistent PSA after SRP was shown to be an independent predictor for BCR and death.
6.4.5.2.1.2. Morbidity
Most reported cases have been open (60%) and robotic-assisted (38%) resulting in an overall complication rate of 34%, with major (Clavien grade ≥ 3) complications occurring across a range 0 to 64% [1067]. Compared to primary open RP, SRP is associated with a higher risk of later anastomotic stricture (47 vs. 5.8%), urinary retention (25.3% vs. 3.5%), urinary fistula (4.1% vs. 0.06%), abscess (3.2% vs. 0.7%) and rectal injury (9.2 vs. 0.6%) [1074]. These complications appear to be less common with robotic compared to open surgery [1066,1068,1075].
Functional outcomes are also worse compared to primary surgery considering urinary incontinence (47.9%, range 21% to 90%) and ED in nearly all patients [1067,1068,1075].
Complications may be lower and functional outcomes may be better with the robotic-assisted approach but certainty of evidence is low [1071].
6.4.5.2.1.3. Summary of salvage radical prostatectomy
In general, SRP should be considered only in patients with low co-morbidity, a life expectancy of at least ten years, a pre-SRP PSA < 10 ng/mL and initial biopsy ISUP grade group ≤ 2/3, localised disease (N0M0) according to re-staging, and those whose initial clinical staging was T1 or T2 [1068].
6.4.5.2.2. Salvage cryoablation of the prostate
6.4.5.2.2.1. Oncological outcomes
Salvage cryoablation of the prostate (SCAP) has been proposed as an alternative to salvage RP, as it has a potentially lower risk of morbidity and equal efficacy.
In a SR a total of 32 studies assessed SCAP, recruiting a total of 5,513 patients. The overwhelming majority of patients (93%) received whole-gland SCAP. The adjusted pooled analysis for 2-year BCR-free survival for SCAP was 67.49% (95% CI: 61.68–72.81%), and for 5-year BCR-free survival was 50.25% (95% CI: 44.10–56.40%). However, the certainty of the evidence was low. Table 6.4.7 summarises the results of a selection of the largest series on SCAP to date in relation to oncological outcomes (BCR only) [1065].
Table 6.4.7: Oncological results of selected salvage cryoablation of the prostate case series, including at least 250 patients
| Oncological results of selected salvage cryoablation of the prostate case series | |||||
| Study | n | Median FU (mo) | Time point of outcome measurement (yr) | BCR-free probability | Definition of failure |
| Ginsburg et al. 2017 [1076] | 898 | 19.0 | 5 yr | 71.3% | Phoenix criteria |
| Spiess et al. 2010 [1077] | 450 | 40.8 | 3.4 yr | 39.6% | PSA > 0.5 ng/mL |
| Li et al. 2015 [1078] | 486 | 18.2 | 5 yr | 63.8% | Phoenix criteria |
| Kovac et al. 2016 [1079] | 486 | 18.2 | 5 yr | 75.5% 22.1% | Phoenix criteria |
| Ahmad et al. 2013 [1080] | 283 | 23.9 | 3 yr | 67.0% 14.0% | Phoenix criteria |
| Pisters et al. 2008 [1081] | 279 | 21.6 | 5 yr | 58.9% (ASTRO) 54.5% (Phoenix) | ASTRO and Phoenix criteria |
ASTRO = American Society for Therapeutic Radiology and Oncology; BCR = biochemical recurrence; FU = follow-up; mo. = months; n = number of patients; PSA = prostate-specific antigen; yr. = year.
6.4.5.2.3. Salvage re-irradiation
6.4.5.2.3.1. Salvage brachytherapy for radiotherapy failure
Carefully selected patients with a good PS, primary localised PCa, good urinary function and histologically proven local recurrence are candidates for salvage BT using either HDR or LDR.
In a SR a total of 16 studies (4 prospective) and 32 studies (2 prospective) assessed salvage HDR and LDR BT, respectively, with the majority (> 85%) receiving whole-gland BT rather than focal treatment [1065]. The adjusted pooled analysis for 2-year BCR-free survival for HDR was 77% (95% CI: 70–83%) and for LDR was 81% (95% CI:74–86%). The 5-year BCR-free survival for HDR was 60% (95% CI: 52–67%) and for LDR was 56% (95% CI: 48–63%). As noted above, BT techniques are associated with lower rates of severe GU toxicity when compared to RP or HIFU, at 8% for HDR (95% CI: 5.1–11%) and 8.1% for LDR (95% CI: 4.3–13%). Rates of severe GI toxicity are reported to be very low at 0% for HDR (95% CI: 0–0.2%) and 1.5% for LDR (95% CI: 0.2–3.4%). High-dose-rate or LDR BT are effective treatment options with an acceptable toxicity profile. However, the published series are small and likely under-report toxicity. Consequently, this treatment should be offered in experienced centres ideally within randomised clinical trials or prospective registry studies (see Table 6.4.8).
Table 6.4.8: Treatment-related toxicity and BCR-free probability in selected salvage brachytherapy studies including at least 100 patients.
| Study | Study design | n and BT type | Median FU (mo) | Treatment toxicity | BCR-free probability |
| Lopez et al. 2019 [1082] | multi-centre retrospective | 75 HDR 44 LDR | 52 | 23.5% late G3+ GU | 5 yr 71% (95% CI: 65.9-75.9%) |
| Crook et al. 2019 [1083] | multi-centre prospective | 100 LDR | 54 | 14% late G3 combined GI/GU | n.r. |
| Smith et al. 2020 [1084] | single-centre retrospective | 108 LDR | 76 | 15.7%/2.8% late G3 GU/GI | 5 yr. 63.1% 10 yr. 52% |
| Lyczek et al. 2009 [1085] | single-centre retrospective | 115 HDR | n.r. | 12.2%/0.9% late G3+ GU/GI | 60% at 40 mo. |
BT = brachytherapy; CI = confidence interval; G = grade; GI = gastro-intestinal; GU = genito-urinary; HDR = high-dose rate; LDR = low-dose rate; mo = months; n = number of patients; n.r. = not reported; yr = year.
6.4.5.2.3.2. Salvage stereotactic ablative body radiotherapy for radiotherapy failure
6.4.5.2.3.2.1.Oncological outcomes and morbidity
Stereotactic ablative body radiotherapy (CyberKnife® or linac-based treatment) is a potentially viable new option to treat local recurrence after RT. Carefully selected patients with good IPSS-score, without obstruction, good PS and histologically proven localised local recurrence are potential candidates for SABR. In a metaanalysis and SR five mostly retrospective studies including 206 patients were treated with CyberKnife® or linac-based treatment showing 2-year RFS estimates (61.6%, 95% CI: 52.6–69.9%) [1065]. In a retrospective multi-centre study (n = 100) the median pre-salvage PSA was 4.3 ng/mL with 34% of patients having received ADT for twelve months (median). All recurrences were biopsy proven. Patients were treated with the CyberKnife® with a single dose of 6 Gy in six daily fractions (total dose 36 Gy). With a median followup of 30 months the estimated 3-year second BCR-free survival was 55% [1086].
In a smaller retrospective series including 50 men with histologically proven local recurrence with a median pre-salvage PSA of 3.9 ng/mL only 15% had received additional ADT. The estimated 5-year second BCR-free survival was 60% (median follow-up of 44 months) which is an outcome comparable to series treating patients with RP, HIFU or BT [1087]. Table 6.4.9 summarises the results of the two larger SABR series addressing oncological outcomes and morbidity.
Table 6.4.9: Treatment-related toxicity and BCR-free survival in selected SABR studies
| Study | Study design | n and RT-type | Median FU (mo) | Fractionation (SD/TD) | ADT | Treatment toxicity | BCR-free survival |
| Bergamin et al. 2020 [1088] | single-centre prospective | 25 LINAC based | 25 | SD 6-6.2 TD 36-38 Gy | 0/25 | 2 yr. late G1 GI 8% G2 GU 4% | 2 yr. 80% |
Fuller et al. 2020 [1087] | single-centre retrospective | 50 Cyber Knife | 44 | SD 6.8 Gy TD 34 Gy | 7/50 | 5 yr: 8% late G3+ GU | 5 yr. 60% |
Pasquier et al. 2020 [1086] | multi-centre retrospective | 100 Cyber Knife | 30 | SD 6 Gy TD 36 Gy | 34/100 median 12 mo. | 3 yr. grade 2+ GU 20.8% GI 1% | 3 yr. 55% |
BCR = biochemical recurrence; FU = follow-up; mo = months; n = number of patients; RT-type = type of radio-therapy; SD = single dose; TD = total dose; yr = year.
6.4.5.2.3.2.2.Morbidity
In a retrospective single-centre study with 50 consecutive patients chronic significant toxicity was only seen for the GU domain with 5-year grade 2+ and grade 3+ GU rates of 17% and 8%, respectively. No GI toxicity > grade 1 was seen. Of note, of the fifteen patients who were sexually potent pre-salvage SBRT, twelve subsequently lost potency [1087]. In a retrospective French (GETUG) multi-centre series (n = 100) the 3-year late grade 2+ GU and GI toxicity was 20.8% (95% CI: 13–29%) and 1% (95% CI: 0.1–5.1%), respectively [1086]. A SR and meta-analysis demonstrated salvage SABR resulted in comparable rates of G3+ GU toxicity when compared to salvage cryotherapy and brachytherapy, but substantially lower rates than salvage HIFU [1089].
6.4.5.2.3.2.3.Summary of salvage stereotactic ablative body radiotherapy
Despite the encouraging results so far the number of patients treated with SABR is relatively limited. In view of the rates of higher grade 2+ GU side effects, SABR should only be offered to selected patients, in experienced centres as part of a clinical trial or well-designed prospective study.
6.4.5.2.4. Salvage high-intensity focused ultrasound
6.4.5.2.4.1. Oncological outcomes
Salvage HIFU has emerged as an alternative thermal ablation option for radiation-recurrent PCa. Being relatively newer than SCAP the data for salvage HIFU are even more limited. A SR and meta-analysis included 20 studies (n = 1,783) assessing salvage HIFU [1065], which was also confirmed by another SR and meta-anaylsis [1089]. The overwhelming majority of patients (86%) received whole-gland salvage HIFU. The adjusted pooled analysis for 2-year BCR-free survival for salvage HIFU was 54.14% (95% CI: 47.77–60.38%) and for 5-year BCR-free survival 52.72% (95% CI: 42.66– 62.56%). However, the certainty of the evidence was low. Table 6.4.10 summarises the results of a selection of the largest series on salvage HIFU to date in relation to oncological outcomes (BCR only).
Table 6.4.10: Oncological results of selected salvage cryoablation of the prostate case series, including at least 250 patients
| Oncological results of selected salvage cryoablation of the prostate case series | |||||
| Study | n | Median FU (mo) | Time point of outcome measurement (yr) | BCR-free probability | Definition of failure |
Crouzet et al. 2017 [1090] | 418 | 39.6 | 5 | 49.0% | Phoenix criteria |
Murat et al. 2009 [1091] | 167 | Mean 18.1 | 3 | 25.0% (high-risk) 53.0% (low-risk)* | Phoenix criteria or positive biopsy or initiation of post-HIFU salvage therapy |
Kanthabalan et al. 2017 [1092] | 150 | 35.0 | 3 | 48.0% | Phoenix criteria |
Jones et al. 2018 [1093] | 100 | 12.0 | 1 | 50.0% | Nadir PSA > 0.5 ng/mL or positive biopsy |
*Results stratified by pre-EBRT D’Amico risk groups.
BCR = biochemical recurrence; FU = follow-up; mo = months; n = number of patients; yr = year.
6.4.5.2.4.2. Morbidity
The main adverse effects and complications relating to salvage HIFU include urinary incontinence, urinary retention due to bladder outflow obstruction, rectourethral fistula and ED. The SR and meta-analysis showed an adjusted pooled analysis for severe GU toxicity for salvage HIFU of 22.66% (95% CI: 16.98–28.85%) [1065]. The certainty of the evidence was low. Table 6.4.11 summarises the results of a selection of the largest series on salvage HIFU to date in relation to GU outcomes.
Table 6.4.11: Peri-operative morbidity, erectile function and urinary incontinence in selected salvage HIFU case series, including at least 100 patients
| Peri-operative morbidity, erectile function and urinary incontinence in selected salvage HIFU case series | ||||||
| Study | n | Time point of outcome measurement (yr) | Incontinence* (%) | Obstruction/ retention (%) | Rectourethral fistula (%) | ED (%) |
| Crouzet et al. 2017 [1090] | 418 | Median 39.6 | 42.3 | 18.0 | 2.3 | n.r. |
| Murat et al. 2009 [1091] | 167 | Median 18.1 | 49.5 | 7.8 | 3.0 | n.r. |
| Kanthabalan et al. 2017 [1092] | 150 | 24 | 12.5 | 8.0 | 2.0 | 41.7 |
| Jones et al. 2018 [1093] | 100 | 12 | 42.0 | 49.0 | 5.0 | 74.0 |
*Incontinence was heterogeneously defined; figures represent at least 1 pad usage.
ED = erectile dysfunction; n.r. = not reported; n = number of patients.
6.4.5.2.4.3. Summary of salvage high-intensity focused ultrasound
There is a lack of high-certainty data which prohibits any recommendations regarding the indications for salvage HIFU in routine clinical practice. There is also a risk of significant morbidity associated with its use in the salvage setting. Consequently, salvage HIFU should only be performed in selected patients in experienced centres as part of a clinical trial or well-designed prospective cohort study.
6.4.6. Hormonal therapy for relapsing patients
The objective of HT should be to improve OS, postpone distant metastases, and improve QoL. Biochemical response alone to HT holds no clinical benefit for a patient. The Panel conducted a SR including studies published from 2000 onwards [1094]. Conflicting results were found on the clinical effectiveness of HT after previous curative therapy. Some studies reported a favourable effect of HT, including the only RCT addressing the research question of this review (86% vs. 79% advantage in OS in the early HT group) [1095]. Other studies did not find any differences between early vs. delayed, or no, HT. One study found an unfavourable effect of HT [1096]. Variability appears to be driven by heterogeneous tumour biology with only a minority progressing to metastases or PCa-related death. For older patients and those with comorbidities the side effects of HT may even decrease life expectancy; in particular cardiovascular risk factors need to be considered [1097,1098]. The benefit of early HT seems most evident in high-risk patients, mainly defined by a high ISUP GG and a short PSA-DT (most often less than six months) and a long-life expectancy [1099].
This is supported in a three-arm randomised phase III trial (EMBARK) which evaluated response in patients with prostate cancer who had high-risk biochemical recurrence defined as a PSA-DT of ≤ 9 months and a PSA level of ≥ 2 ng/mL above the nadir after radiation therapy or ≥ 1 ng/mL after radical prostatectomy with or without postoperative radiation therapy [1100]. Patients were randomly assigned 1:1:1 to receive enzalutamide daily plus leuprolide every 12 weeks (combination group), placebo plus leuprolide (leuprolide-alone group), or enzalutamide monotherapy (monotherapy group). The primary end point was MFS, in the combination group as compared with the leuprolide- alone group. The MFS in the monotherapy group as compared with the leuprolide-alone group was a key secondary endpoint. A total of 1068 patients were randomised. After a median follow-up of 60.7 months, the five year - MFS was 87.3% (95% CI, 83.0 - 90.6) in the combination group, 71.4% (95% CI, 65.7 - 76.3) in the leuprolide-alone group, and 80.0% (95% CI, 75.0 - 84.1) in the monotherapy group. The combination of enzalutamide plus leuprolide was superior to leuprolide alone with regards to the MFS (HR 0.42; 95% CI, 0.30 - 0.61; P<0.001). Enzalutamide monotherapy also showed a superior MFS compared to leuprolide alone (HR 0.63; 95% CI, 0.46 - 0.87; p = 0.005). These results led to the FDA approval for enzalutamide alone or in combination with ADT for patients with high-risk biochemical recurrence in November 2023 [1101]. At the time of the MFS analysis, OS data were immature with 12% deaths in the overall population.
Also, an intermittent treatment approach can be considered. Enzalutamide treatment can be suspended if PSA is undetectable (< 0.2 ng/mL) after 36 weeks of therapy. Treatment may be reinitiated when PSA has increased to ≥ 2.0 ng/mL for patients who had prior radical prostatectomy or ≥ 5.0 ng/mL for patients who had prior primary radiation therapy. There were no new safety signals. Of note, at a median follow-up of five years, the overall percentage of patients who had fractures was 14% [1102].
Another three-arm-randomised phase-III trial (PRESTO) evaluated patients with biochemical recurrence defined as a PSA-DT < 9 months with a median PSA of 1.8 ng/mL [1103]. Patients were randomly assigned 1:1:1 to receive (52-week treatment) ADT-control, ADT + apalutamide, or ADT + apalutamide + Abiraterone acetate plus prednisolone (AAP). A total of 503 patients were randomised. At the first interim analysis after a median follow up of 21.5 months both experimental arms showed a moderate, significant prolonged PSA-PFS compared with the control arm (24.9 months for ADT + apalutamide versus 20.3 months for ADT (HR 0.52, p = 0.00047 and 26 months for ADT + apalitamide + AAP versus 20.0 months for ADT (HR 0.48, p = 0.00008). The most common grade > 3 AE was hypertension (7.5%, 7.4% and 18% in ADT, ADT + apalutamide and ADT + apalutamide + AAP). These are results of the first planned interim analysis and longer follow up for definitive conclusions should be awaited.
A Scandinavian Phase-III-trial (SPCG-14) [1104] evaluated the effect of docetaxel added to bicalutamide in hormone-naïve non-metastatic PCa with a rising PSA after radical treatment (prostatectomy or radiotherapy, n = 315) or not suitable for curative treatment (n = 3). Between 2009 and 2018 348 patients were randomized, median follow up was 4.9 years. Adding docetaxel improved PFS (HR 0.68, p = 0.015) at the cost of 27% of one event of neutropenic infection/fever. There were no data on metastasis-free-survival. It is therefore too early to consider recommending at this time no recommendation for adding docetaxel in this setting of PSA-recurrence only can be given.
6.4.7. Observation
In unselected relapsing patients the median actuarial time to the development of metastasis will be eight years and the median time from metastasis to death will be a further five years [884]. For patients with EAU Low-Risk BCR features, unfit patients with a life expectancy of less than ten years or patients unwilling to undergo salvage treatment, active follow-up may represent a viable option.
6.4.8. Recommendations for second-line therapy after treatment with curative intent
| Local salvage treatment | Strength rating |
| Recommendations for biochemical recurrence (BCR) after radical prostatectomy | |
| Offer early salvage intensity-modulated radiotherapy/volumetric arc radiation therapy plus image-guided radiotherapy to men with two consecutive prostate-specific antigen (PSA) rises. | Strong |
| A negative positron emission tomography/computed tomography (PET/CT) scan should not delay salvage radiotherapy (SRT), if otherwise indicated. | Strong |
| Offer monitoring, including PSA, to EAU low-Risk BCR patients. | Weak |
| Do not wait for a PSA threshold before starting treatment. Once the decision for SRT has been made, SRT (at least 64 Gy) should be given as soon as possible. | Strong |
| Offer hormonal therapy in addition to SRT to men with BCR. | Weak |
| Recommendations for BCR after radiotherapy | |
| Offer monitoring, including PSA to EAU low-risk BCR patients. | Weak |
| Only offer salvage radical prostatectomy (RP), brachytherapy, stereotactic body radiotherapy, high-intensity focused ultrasound, or cryosurgical ablation to highly selected patients with biopsy-proven local recurrence within a clinical trial setting or well-designed prospective cohort study undertaken in experienced centres. | Strong |
| Recommendations for systemic salvage treatment | |
| Do not offer androgen deprivation therapy to M0 patients with a PSA-doubling time > 12 months. | Strong |
| Offer enzalutamide with or without ADT to M0 patients with a high-risk BCR , defined as a PSA doubling time of ≤ 9 months and a PSA level of ≥ 2ng/mL above nadir after radiation therapy or ≥ 1 ng/m after radical prostatectomy with or without postoperative radiation therapy. | Strong |
| Recommendations for follow-up after radical prostatectomy or radiotherapy | |
| Routinely follow-up asymptomatic patients by obtaining at least a disease-specific history and serum PSA measurement. | Strong |
| At recurrence, only perform imaging if the result will affect treatment planning. | Strong |
6.5. Systemic treatments for prostate cancer
6.5.1. Hormonal therapy
Androgen deprivation can be achieved by suppressing the secretion of testicular androgens in different ways.
6.5.1.1. Castration level
The castration level of testosterone is < 50 ng/dL (1.7 nmol/L), defined more than 40 years ago when testosterone testing was less sensitive. Current methods have shown that the mean value after surgical castration is 15 ng/dL [1035]. Therefore, a more appropriate level should be defined as < 20 ng/dL (< 0.7 nmol/L). This definition is important as better results are repeatedly observed in ADT monotherapy cohorts with lower testosterone levels compared to 50 ng/dL [1036-1038]. However, the castrate level considered by the regulatory authorities and in clinical trials addressing castration in PCa is still the historical < 50 ng/dL (1.7 nmol/L).
6.5.1.2. Bilateral orchiectomy
Bilateral orchiectomy or subcapsular pulpectomy is still considered the primary treatment modality for ADT. It is a simple, cheap and low-complication procedure. It is easily performed under local anaesthesia, and it is the quickest way to achieve a castration level which is usually reached within less than twelve hours. It is irreversible and therefore does not allow for intermittent treatment [1039].
6.5.1.3. Luteinising-hormone-releasing hormone agonists
Long-acting LHRH agonists are currently the main forms of ADT. These synthetic analogues of LHRH are delivered as depot injections on a 1-, 3-, 6-monthly, or yearly basis. The first injection induces a transient rise in luteinising hormone (LH) and follicle-stimulating hormone (FSH) leading to the ‘testosterone surge’ or ‘flare-up’ phenomenon which starts two to three days after administration and lasts for about one week. This may lead to detrimental clinical effects (the clinical flare) such as increased bone pain, acute bladder outlet obstruction, obstructive renal failure, spinal cord compression, and cardiovascular death due to hypercoagulation status [1105]. Patients at risk are usually those with high-volume symptomatic bony disease. Concomitant therapy with an anti-androgen decreases the incidence of clinical flare but does not completely remove the risk. Anti-androgen therapy is usually continued for 4 weeks but neither the timing nor the duration of anti-androgen therapy are based on strong evidence. In addition, the long-term impact of preventing ‘flare up’ is unknown [1106].
Chronic exposure to LHRH agonists results in the down-regulation of LHRH-receptors, suppressing LH and FSH secretion and therefore testosterone production. A castration level is usually obtained within 2 to 4 weeks [1107]. Although there is no formal direct comparison between the various compounds, they are considered to be equally effective [1108]. So far, no survival difference between LHRH agonists and orchiectomy has been reported due to the lack of high-quality trials [1109]. The different products have practical differences that need to be considered in everyday practice, including the storage temperature, whether a drug is ready for immediate use or requires reconstitution, and whether a drug is given by subcutaneous or intramuscular injection.
6.5.1.4. Luteinising-hormone-releasing hormone antagonists
Luteinising-hormone-releasing hormone antagonists immediately bind to LHRH receptors, leading to a rapid decrease in LH, FSH and testosterone levels without any flare. The practical shortcoming of these compounds is the lack of a long-acting depot formulation with, so far, only monthly formulations being available. Degarelix is a LHRH antagonist. The standard dosage is 240 mg in the first month followed by monthly injections of 80 mg. Most patients achieve a castrate level at day three [1107]. A phase III RCT compared degarelix to monthly leuprorelin following up patients for twelve months, suggesting a better PSA PFS for degarelix 240/80 mg compared to monthly leuprorelin [1110]. A SR did not show a major difference between agonists and degarelix and highlighted the paucity of on-treatment data beyond twelve months as well as the lack of survival data [1111]. Its definitive superiority over the LHRH analogues remains to be proven. Short-term follow-up data from a meta-analysis indicate that the use of LHRH antagonist is associated with significantly lower overall mortality and cardiovascular events as compared with agonists. On the other hand, other adverse effects such as decreased libido, hot flushes, ED, weight gain, and injection site reactions are seen less often with the agonists [1112,1113].
Relugolix is an oral LHRH antagonist. It was compared to the LHRH agonist leuprolide in a randomised phase III trial [1114]. The primary endpoint was sustained testosterone suppression to castrate levels through 48 weeks. There was a significant difference of 7.9 percentage points (95% CI: 4.1–11.8) showing non-inferiority and superiority of relugolix. The incidence of major adverse cardiovascular events was significantly lower with relugolix (prespecified safety analysis). Relugolix has been approved by the FDA [1115] and EMA [1116] for hormone sensitive PCa.
6.5.1.5. Anti-androgens
These oral compounds are classified according to their chemical structure as:
- steroidal, e.g., cyproterone acetate (CPA), megestrol acetate and medroxyprogesterone acetate;
- non-steroidal or pure, e.g., nilutamide, flutamide and bicalutamide.
Both classes compete with androgens at the receptor level. This leads to an unchanged or slightly elevated testosterone level. Conversely, steroidal anti-androgens have progestational properties leading to central inhibition by crossing the blood-brain barrier.
6.5.1.5.1. Steroidal anti-androgens
These compounds are synthetic derivatives of hydroxyprogesterone. Their main pharmacological side effects are secondary to castration (gynaecomastia is quite rare) whilst the non-pharmacological side effects are cardiovascular toxicity (4–40% for CPA) and hepatotoxicity.
Cyproterone acetate was the first licensed anti-androgen but the least studied. Its most effective dose as monotherapy is still unknown. It appears to be associated with a poorer OS when compared with LHRH analogues and there is no benefit when compared with flutamide [1117,1118].
6.5.1.5.2. Non-steroidal anti-androgens
Non-steroidal anti-androgen monotherapy with e.g. nilutamide, flutamide or bicalutamide does not suppress testosterone secretion and it is claimed that libido, overall physical performance and bone mineral density (BMD) are frequently preserved [1119]. Non-androgen-related pharmacological side effects differ between agents. Bicalutamide shows a more favourable safety and tolerability profile than flutamide and nilutamide [1120]. The dosage licensed for use in combination with LHRH blockade is 50 mg/day, and 150 mg/day for monotherapy. The androgen pharmacological side effects are mainly gynaecomastia (70%) and breast pain (68%). However, non-steroidal anti-androgen monotherapy offers clear bone protection compared with LHRH analogues and probably LHRH antagonists [1119,1121]. All three agents share the potential for liver toxicity (occasionally fatal), requiring regular monitoring of patients’ liver enzymes.
6.5.1.5.3. New androgen receptor pathway inhibitors (ARPIs)
Once on ADT the development of castration-resistance (CRPC) is only a matter of time. It is considered to be mediated through two main overlapping mechanisms: androgen-receptor (AR)-independent and AR-dependent mechanisms. In CRPC, the intracellular androgen level is increased compared to androgen sensitive cells and an over-expression of the AR has been observed, suggesting an adaptive mechanism [1122]. This has led to the development of several compounds targeting the androgen axis. The status of the different ARPIs is summarised in table 6.5.1 [1123-1128]. For the updated approval status see EMA and FDA websites [1101,1129-1132].
Table 6.5.1: Status of the different ARPIs
| Status of the different ARPIs | |||||
| High-risk localised & locally advanced** | High-risk BCR | mHSPC | nmCRPC | mCRPC | |
| Abiraterone | X* | X | X | ||
| Enzalutamide | X | X | X | X | |
| Apalutamide | X | X | |||
| Darolutamide | X | X | |||
* Unlicenced indication
** STAMPEDE definition
6.5.1.5.3.1. Abiraterone acetate
Abiraterone acetate is a CYP17 inhibitor (a combination of 17α-hydrolase and 17,20-lyase inhibition). By blocking CYP17, abiraterone acetate significantly decreases the intracellular testosterone level by suppressing its synthesis at the adrenal level and inside the cancer cells (intracrine mechanism). This compound must be used together with prednisone/prednisolone to prevent drug-induced hyperaldosteronism [1129,1131].
6.5.1.5.3.2. Apalutamide, darolutamide, enzalutamide and rezvilutamide (alphabetical order)
These agents are novel non-steroidal anti-androgens with a higher affinity for the AR receptor than traditional non-steroidal anti-androgens. In addition, while previous non-steroidal anti-androgens still allow transfer of ARs to the nucleus and would act as partial agonists, all four agents also block AR transfer and therefore suppress any possible agonist-like activity [1123,1124,1132,1133]. Darolutamide has structurally unique properties; in particular, in preclinical studies, it was shown not to cross the blood-brain barrier [1134,1135].
6.5.2. Cytotoxic drug treatment
6.5.2.1. Taxanes
Paclitaxel derivatives promote the assembly of microtubules and inhibit the subsequent depolymeization, impairing the tubulin dynamics that foster the mitotic spindle assembly during interphase in mitosis [1136]. Docetaxel binds ß-tubulin dimers in a 1:1 stoichiometric ratio, exhibiting a stronger dynamic instability using its inhibitory effect in tubulin depolymerization [1137]. It also activates NF-kB causing apoptosis via a mitochondria-dependent pathway [1138]. Docetaxel shows significant activity against prostate tumours. Cabazitaxel also works by binding to the microtubules. This prevents cellular mitosis and stabilises the tumour cells. As a result, the cells do not divide. In addition, it inhibits androgen receptors by binding to the microtubules and microtubule-associated motor protein dynein. As a consequence, androgen receptor nuclear translocation is prevented [1136]. Common side-effects include peripheral neuropathy, myalgias, neutropenia and arthralgia.
6.5.3. Non-hormonal non-cytotoxic drug treatments
6.5.3.1. Poly (ADP-ribose) polymerase inhibitirs (PARPi)
Poly (ADP-ribose) polymerase inhibitors (PARPi) block the enzyme poly ADP-ribose polymerase (PARP) and were developed aiming to selectively target cancer cells harbouring BRCA mutations and other mutations inducing homologous recombination deficiency and high level of replication pressure with a sensitivity to PARPi treatment. Due to the oncogenic loss of some DNA repair effectors and incomplete DNA repair repertoire, some cancer cells are addicted to certain DNA repair pathways such as Poly (ADP-ribose) polymerase (PARP)-related single-strand break repair pathway. The interaction between BRCA and PARP is a form of synthetic lethal effect which means the simultaneously functional loss of two genes leads to cell death, while a defect in any single gene only has a limited effect on cell viability [1139]. BRCA mutations both predispose patients to develop PCa and develop in some tumours making some patients particularly responsive to these drugs.
6.5.3.2. Immune checkpoint inhibitors
Checkpoint inhibitors target the molecules CTLA4, programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1). For advanced PCa patients that are microsatellite instability-high/deficient mismatch repair (MSI-H/dMMR), the PD-1 inhibitor pembrolizumab has been approved by the FDA but not by the EMA. The label is tumour agnostic [1140,1141].
6.5.3.3. Radiopharmaceutical therapy
Radiopharmaceutical therapy (RPT) is based on the delivery of radioactive isotopes to tumour-associated targets. The mechanism of action for RPT is radiation-induced killing of cells. Radionuclides with different emission properties are used to deliver radiation. The most commonly used radionuclides are represented by β-particles (e.g., 177Lu) or α-particles (e.g., 223Ra, 225Ac). 223Ra based on its biochemical similarity to Calcium, is integrated in bones with increased osteoblastic activity, thus targeting skeletal PCa metastases. 177Lu is increasingly used because of its optimal imaging range (100–200 keV), favourable half time (6.6 days) and appropriate β-particle energy for therapy. The short path of the β-particles (0.05–0.08 mm) results in minimal toxic effects in adjacent healthy tissue. These properties enable such radionuclides to be used as theranostics (i.e., the same radionuclide may be used for both diagnostic and therapeutic purposes). However, an essential requirement prior to any RPT is to assess the targeting of the agent, mainly using PET techniques which show the tumour expression and the extent of cancer [1142]. 177Lu has been approved by the FDA for the treatment of adult patients with PSMA-positive mCRPC who have been treated with ARPI and taxane-based chemotherapy [1143,1144].
6.6. Management of Metastatic prostate cancer
6.6.1. Introduction
Most prospective data available rely on the definition of M1 disease based on CT scan or MRI and bone scintigraphy. The influence on treatment and outcome of newer, more accurate, imaging has not yet been assessed in prospective randomised trials.
6.6.2. Prognostic and predictive factors
Median survival of patients with newly diagnosed metastases (synchronous mHSPC) is approximately 50 months with ADT alone, however, it is highly variable since the M1 population is heterogeneous [1145]. Several prognostic factors for survival have been suggested including the number and location of bone metastases, presence of visceral metastases, ISUP GG, performance status and initial PSA and alkaline phosphatase level, but only few have been validated [1146-1149].
‘Volume‘ of disease as a potential predictor was introduced by CHAARTED (Chemo-hormonal Therapy versus Androgen Ablation Randomised Trial for Extensive Disease in Prostate Cancer) [1149-1151] (Table 6.6.1) and subsequently, in STAMPEDE, was shown to be predictive in an adequately powered subgroup analysis for benefit of addition of prostate RT to ADT in the subgroup of patients with low volume/burden disease [1152] (Table 6.6.1).
‘Metachronous’ metastatic disease (after radical local treatment of the primary tumour) vs. synchronous (or de novo) metastatic disease has also been shown to have generally a better prognosis [1153].
Based on a large SWOG 9346 cohort, the PSA level after seven months of ADT was used to create three prognostic groups (Table 6.6.2) [1154]. A PSA ≤ 0.2 ng/mL at seven months has been confirmed as a prognostic marker for men receiving ADT for metastatic disease in the CHAARTED study independent of the addition of docetaxel [1155]. Similarly, reaching PSA levels of ≤ 0.1ng/ml after six months were shown to be correlated with long-term outcomes in the LATITUDE study [1156]. Also for patients treated with ADT and apalutamide a deep PSA decline defined by ≥ 90% from baseline or to PSA ≤ 0.2 ng/mL at a landmark of three months was associated with longer OS [1157] for patients.
Table 6.6.1: Definition of high- and low-volume in CHAARTED [1149-1151] and high- and low-risk in LATITUDE [1127]
| High | Low | |
| CHAARTED (volume) | > 4 Bone metastases including > 1 outside vertebral column or pelvis AND/OR Visceral metastasis* | Not high |
LATITUDE (risk) | > 2 high-risk features of: > 3 Bone metastasis Visceral metastasis > ISUP grade 4 | Not high |
*Lymph nodes are not considered as visceral metastases.
Table 6.6.2: Prognostic factors based on the SWOG 9346 study [1154]
| PSA after 7 months after start of ADT | Median survival on ADT monotherapy |
| < 0.2 ng/mL | 75 months |
| 0.2 < 4 ng/mL | 44 months |
| > 4 ng/mL | 13 months |
6.6.3. First-line hormonal treatment
Primary ADT has been the SOC for over 50 years [1158]. There is no high-level evidence in favour of a specific type of ADT for oncological outcomes, neither for orchiectomy nor for a LHRH agonist or antagonist. The level of testosterone is reduced much faster with orchiectomy and LHRH antagonist, therefore patients with impending spinal cord compression or other potential impending complications from the cancer should be treated with either a bilateral orchidectomy or LHRH antagonists as the preferred options.
There is a suggestion in some studies and a SR and meta-analysis that cardiovascular side effects are less frequent in patients treated with LHRH antagonists than patients treated with LHRH agonists [1114,1159-1161]; therefore, patients with pre-existing cardiovascular disease or other cardio-vascular risk factors might be considered to be treated with antagonists if a chemical castration is chosen.
6.6.3.1. Non-steroidal anti-androgen monotherapy
Based on a Cochrane review comparing older generation non-steroidal anti-androgen (NSAA) monotherapy to ADT (either medical or surgical), NSAA was considered to be less effective in terms of OS, clinical progression, treatment failure and treatment discontinuation due to AEs [1162] and is generally not recommended also because ADT-based combination treatments have become SOC.
6.6.3.2. Intermittent versus continuous androgen deprivation therapy
Three independent reviews [1163-1165] and two meta-analyses [1166,1167] looked at the clinical efficacy of intermittent androgen deprivation (IAD) therapy. All of these reviews included 8 RCTs of which only three were conducted in patients with exclusively M1 disease.
So far, the SWOG 9346 is the largest trial addressing IAD in M1b patients [1168]. Of 3,040 screened patients, only 1,535 patients met the inclusion criteria. This highlights that only about 50% of M1b patients can be expected to be candidates for IAD, i.e. the best PSA responders. This was a non-inferiority trial leading to inconclusive results: the actual upper limit was above the pre-specified 90% upper limit of 1.2 (HR: 1.1, CI: 0.99–1.23), the pre-specified non-inferiority limit was not achieved, and the results did not show a significant inferiority for any treatment arm. However, based on this study inferior survival with IAD cannot be completely ruled out even in this highly selected subgroup. The use of intermittent ADT has been superseded as continuous ADT based combination therapy has become SOC.
6.6.3.3. Early versus deferred androgen deprivation therapy
Early treatment before the onset of symptoms is recommended in the majority of patients with metastatic hormone-sensitive disease. A Cochrane analysis from 2019 about the topic concluded that early ADT probably extends time to death of any cause and time to death from PCa [1169], but the analysis included only a very limited number of metastatic patients. There is a lack of randomised phase III data in this specific setting and specifically not with the combination therapies that are standard nowadays, however data is accumulating for the use of long-term ADT earlier in the disease pathway.
The addition of RT/ SABR to ADT monotherapy or combination with ARPI as well as the use of SABR to delay ADT is discussed in section 6.6.7.
6.6.4. Combination therapies
All of the following combination therapies have been studied with continuous ADT, not intermittent ADT.
6.6.4.1.‘Combined’ androgen blockade with older generation NSAA (bicalutamide, flutamide, nilutamide)
Systematic reviews have shown that combined androgen blockade using a NSAA appears to provide a small survival advantage (< 5%) vs. monotherapy (surgical castration or LHRH agonists) [1170,1171]. This minimal survival advantage must be balanced against the increased side effects especially as the newer combination therapies are more effective as shown specifically for enzalutamide which was tested against NSAA in a phase III trial [1172]. More recently another trial has demonstrated a significant OS benefit for the addition of rezvilutamide vs. addition of bicalutamide to ADT in patients with high-volume mHSPC [1173]. Therefore, combination with NSAAs should only be considered if other combination therapies are not available.
6.6.4.2. Androgen deprivation combined with other agents
6.6.4.2.1. Combination with an ARPI alone (abiraterone, apalutamide, enzalutamide, rezvilutamide, darolutamide)
In two large RCTs (STAMPEDE, LATITUDE) the addition of abiraterone acetate (1000 mg daily) plus prednisone (5 mg daily) to ADT in men with mHSPC was studied [1127,1174,1175] (Table 6.6.3). The primary objective of both trials was an improvement in OS. Both trials showed a significant OS benefit. In LATITUDE with only de novo high-risk metastatic patients included, the HR reached 0.62 (0.51–0.76) [1127]. The HR in STAMPEDE was very similar with 0.63 (0.52–0.76) in the total patient population (metastatic and non-metastatic) and a HR of 0.61 in the subgroup of metastatic patients [1174]. While only high-risk patients were included in the LATITUDE trial a post-hoc analysis from STAMPEDE showed the same benefit whatever the risk or the volume category was [1176].
All secondary objectives such as PFS, time to radiographic progression, time to pain, or time to chemotherapy were in favour of the combination. No difference in treatment-related deaths was observed with the combination of ADT plus AAP compared to ADT monotherapy (HR: 1.37 [0.82–2.29]). However, twice as many patients discontinued treatment due to toxicity in the combination arms in STAMPEDE (20%) compared to LATITUDE (12%) [1175]. Based on these data upfront AAP combined with ADT should be considered as a standard in men presenting with metastases at first presentation, provided they are fit enough to receive the drug.
In five large RCTs the addition of AR antagonists to ADT in men with mHSPC was tested [1125,1126,1172]. In ARCHES the primary endpoint was radiographic PFS (rPFS). In the primary analysis rPFS was significantly improved for the combination of enzalutamide and ADT with a HR of 0.39 (0.3–0.5). Approximately 36% of the patients had low-volume disease; around 25% had prior local therapy and 18% of the patients had received prior docetaxel. In the final prespecified analysis the key secondary enpoint OS was significantly improved with a HR of 0.66 (0.53-0.81) and a significant benefit for rPFS was maintained with a HR of 0.63 (0,52–0.76) [1177].
In ENZAMET the primary endpoint was OS. The addition of enzalutamide to ADT in the first analysis improved OS with a HR of 0.67 (0.52–0.86) compared to ADT plus a non-steroidal antiandrogen. Approximately half of the patients had concomitant docetaxel; about 40% had prior local therapy and about half of the patients had low-volume disease [1126]. In a planned later analysis with a median follow-up of 68 months the OS benefit of adding enzalutamide was maintained with a HR of 0.7 (0.58-0.84) (Table 6.6.4) [1178].
In the TITAN trial, ADT plus apalutamide was used and rPFS and OS were co-primary endpoints. In the primary analysis rPFS was significantly improved by the addition of apalutamide with a HR of 0.48 (0.39–0.6); OS at 24 months was improved for the combination with a HR of 0.67 (0.51–0.89). In the final analysis the HR for OS was 0.65 (0.53–0.79) without adjustment for cross-over. In this trial 16% of patients had prior local therapy, 37% had low-volume disease and 11% received prior docetaxel [1125,1179] (Table 6.6.4). A secondary analysis of the Titan study found that nearly half of the patients developing subsequent radiographic progression had no concomitant PSA progression, suggesting that heavy reliance on PSA monitoring may be inadequate for assessing disease activity in this context [1180].
In the CHART trial, ADT plus rezvilutamide was tested vs. ADT plus bicalutamide in patients with high-volume de novo metastatic disease. Ninety percent of the patients were recruited in China. Overall survival and rPFS were co-primary endpoints. At the pre-planned interim analysis rezvilutamide significantly improved rPFS compared with bicalutamide with a HR of 0.44 (0.33–0.58) and OS with a HR of 0.58 (0.44–0.77) (Table 6.6.5) [1173].
In ARANOTE, darolutamide plus ADT was randomised 2:1 vs. placebo plus ADT. It showed to significantly improved rPFS which was the primary endpoint (HR 0.54 [95% CI, 0.41 to 0.71]; p < 0.0001), with consistent benefits across subgroups, including high- and low-volume disease [1128]. Adverse events were similar in the two groups. Overall survival was not statistically different, but data are immature (HR, 0.81 [95% CI, 0.59 to 1.12]) (Table 6.6.6).
In summary, the addition of the new AR antagonists significantly improves clinical outcomes with no convincing evidence of differences between subgroups. The majority of patients had de novo metastatic disease but a proportion of patients had metachronous disease; in the subgroup analyses the effect seemed to be consistent and therefore, a combination should also be offered for men progressing after radical local therapy [1178,1181,1182].
Table 6.6.3: Results from the STAMPEDE arm G and LATITUDE studies
| STAMPEDE [1174] | LATITUDE [1127] | |||
| ADT | ADT + AA + P | ADT + placebo | ADT + AA + P | |
| N | 957 | 960 | 597 | 602 |
| Newly diagnosed N+ | 20% | 19% | 0 | 0 |
| Newly diagnosed M+ | 50% | 48% | 100% | 100% |
| Key inclusion criteria | Patients scheduled for long-term ADT
or PSA > 20 ng/mL | Newly diagnosed M1 disease and 2 out of the 3 risk factors: ISUP GG ≥ 4, ≥ 3 bone lesions, measurable visceral metastasis | ||
| Primary objective | OS | OS; rPFS | ||
| Median follow up | 40 mo | 30.4 mo | ||
| 3-yr. OS | 83% (ADT + AA + P) 76% (ADT) | 66% (ADT + AA + P) 49% (ADT + placebo) | ||
| HR (95% CI) | 0.63 (0.52-0.76) | 0.62 (0.51-0.76) | ||
| M1 only | ||||
| N | 1,002 | 1,199 | ||
| 3-yr. OS | NA | 66% (ADT + AA + P) 49% (ADT + placebo) | ||
| HR (95% CI) | 0.61 (0.49-0.75) | 0.62 (0.51-0.76) | ||
| HR | FFS (biological, radiological, clinical or death): 0.29 (0.25-0.34) | rPFS: 0.49 (0.39-0.53) | ||
AA = abiraterone acetate; ADT = androgen deprivation therapy; CI = confidence interval; FFS = failure-freesurvival; HR = hazard ratio; ISUP = International Society of Urological Pathology; mo = month; n = number of patients; NA = not available; OS = overall survival; P = prednisone; rPFS = radiographic progression-free survival; PSA = prostate-specific antigen; yr. = year.
Table 6.6.4: Results from the ENZAMET and TITAN studies with OS as primary endpoint
| ENZAMET [1172,1178] | TITAN [1125,1179] | |||
| ADT+ older antagonist ± docetaxel (SOC) | ADT + enzalutamide ± docetaxel | ADT + placebo | ADT + apalutamide | |
| N | 562 | 563 | 527 | 525 |
| Newly diagnosed M+ | 72.1% | 72.5% | 83.7% | 78.3% |
| Low volume | 47% | 48% | 36% | 38% |
| Primary objective | OS | OS; rPFS | ||
| Median follow up (mo) | 68 mo | 30.4 mo | ||
| OS | 5-year survival: 67% (ADT + enzalutamide) 57% (SOC) | 2-yr survival: 84% (ADT + apalutamide) 74% (ADT + placebo) | ||
| HR (95% CI) for OS | 0.70 (0.58–0.84) | 0.67 (0.51-0.89) | ||
ADT = androgen deprivation therapy; CI = confidence interval; HR = hazard ratio; mo = month; n = number of patients; OS = overall survival; SOC = standard of care; rPFS = radiographic progression-free survival; yr = year.
Table 6.6.5: Results from the ARCHES and CHART studies
| ARCHES [1126,1177] | CHART [1173] | |||
| ADT ± docetaxel | ADT + enzalutamide ± docetaxel | ADT + bicalutamide | ADT + rezvilutamide | |
| N | 576 | 574 | 328 | 326 |
| Newly diagnosed M+ | 63% | 70% | 100% | 100% |
| Low volume | 35% | 38% | 0% | 0% |
| Use of early docetaxel | 18% (previous) | 18% (previous) | 0% | 0% |
| Primary endpoint(s) | rPFS | OS; rPFS | ||
| Median follow up | 44.6 mo | 29.3 mo | ||
| Median rPFS (mo.) | 38.9 mo | 49.8 mo | 23.5 mo | Not reached |
| HR (95% CI) for rPFS | HR: 0.63 (0.52–0.76) | HR: 0.46 (0.36–0.60) | ||
| Median OS | Not reached | Not reached | Not reached | Not reached |
| HR (95% CI) for OS | 0.66 (0.53–0.81): Main secondary endpoint | 0.58 (0.44–0.77) | ||
HR = hazard ratio; mo = month; n = number of patients; OS = overall survival; rPFS = radiographic progression-free survival; yr = year.
Table 6.6.6: Results from ARANOTE study with rPFS as the first endpoint
| ARANOTE [1128] | ||
| ADT+darolutamide | ADT + placebo | |
| N | 448 | 223 |
| Newly diagnosed M+ | 71.1% | 75.3% |
| Low volume | 29.4% | 29.6% |
| Use of early docetaxel | 0 | 0 |
| Primary endpoint(s) | rPFS | |
| Median follow-up | 25.3 mo | 25.0 mo |
| Median rPFS | Not reached | 25.0 mo |
| HR (95% CI) for rPFS | 0.54 (0.41 - 0.71); P < .0001) | |
| Median OS | Not reached | Not reached |
| HR (95% CI) for OS | 0.81 (0.59 - 1.12]): immature | |
HR = hazard ratio; mo = month; OS = overall survival; rPFS = radiographic progression-free survival.
6.6.4.2.2. Androgen deprivation therapy combined with chemotherapy
Three large RCTs were conducted [775,1088,1114]. All trials compared ADT alone as the SOC with ADT combined with immediate docetaxel (75 mg/sqm, every three weeks within three months of ADT initiation). The primary objective in all three studies was to assess OS.
In the GETUG 15 trial, all patients had M1 PCa, either de novo or after a primary treatment [1183]. They were stratified based on previous treatment and Glass risk factors [1146]. In the CHAARTED trial the same inclusion criteria applied, and patients were stratified according to disease volume [1149].
STAMPEDE is a multi-arm multi-stage trial in which the reference arm (ADT monotherapy) included 1,184 patients. One of the experimental arms was docetaxel combined with ADT (n = 593), another was docetaxel combined with zoledronic acid (n = 593). Patients were included with either M1 or N1 or having two of the following 3 criteria: T3/4, PSA ≥ 40 ng/mL or ISUP grade group 4–5. Also relapsed patients after local treatment were included if they met one of the following criteria: PSA ≥ 4 ng/mL with a PSA-DT < six months or a PSA ≥ 20 ng/mL, N1 or M1. No stratification was used regarding metastatic disease volume (high/low volume) [839]. In all 3 trials toxicity was mainly haematological with around 12–15% grade 3–4 neutropenia, and 6–12% grade 3–4 febrile neutropenia. The use of granulocyte colony-stimulating factor receptor (GCSF) was shown to be beneficial in reducing febrile neutropenia. Primary or secondary prophylaxis with GCSF should be based on available guidelines [1184,1185].
Docetaxel in all three trials was used at the standard dose of 75 mg/sqm every three weeks, 6 cycles in CHAARTED and STAMPEDE and up to 9 cycles in GETUG-AFU-15. In subgroup analyses from GETUG-AFU 15 and CHAARTED the beneficial effect of the addition of docetaxel to ADT was most evident in men with de novo metastatic high-volume disease [1150,1151], while it was in the same range whatever the volume in the post-hoc analysis from STAMPEDE [1186]. The effect of adding docetaxel was less apparent in men who had prior local radical treatment although the numbers were small and the event rates low. A SR and meta-analysis which included these 3 trials showed that the addition of docetaxel to SOC improved survival [1185]. The HR of 0.77 (95% CI: 0.68–0.87, p < 0.0001) translates into an absolute improvement in four-year survival of 9% (95% CI: 5–14). In a SR and meta-analysis of individual participant data from the three trials it has been shown that there is no meaningful beneficial effect of addition of docetaxel to ADT for patients with metachronous low volume disease. Interestingly the largest absolute improvement at five years was observed for the patients with high volume and clinical stage 4 disease [1187]. Therefore adding docetaxel alone to ADT should only be considered if no ARPI is available or all available ARPIs are contraindicated.
The addition of abiraterone to ADT and docetaxel has been reported to have a benefit in rPFS and in OS in the PEACE-1 trial [1188,1189]. The trial has a 2x2 factorial design and participants with de novo (synchronous) metastatic PCa were randomised to SOC; at the beginning of the trial ADT, later ADT plus docetaxel for 6 cycles if chemotherapy-fit) vs. SOC plus radiotherapy vs. SOC plus abiraterone vs. SOC plus radiotherapy plus abiraterone. Co-primary endpoints were rPFS and OS, both were statistically significantly improved in the total population. Also in the group of patients who received ADT plus docetaxel as SOC (n = 710) both rPFS and OS were increased with a HR: 0.5 (0.34–0.71) and 0.75 (0.59–0.95), respectively. Of note; in this population about 35% had low-volume disease. Toxicity was modestly increased, mostly hypertension.
In the ARASENS Phase III trial all patients received ADT and docetaxel for 6 cycles as SOC plus darolutamide or placebo [1190]. 1,306 metastatic patients were included, 14 % of them with relapsed disease after radical local treatment (metachronous). Primary endpoint was OS and this was statistically significantly increased by the addition of darolutamide with a HR of 0.68 (0.57–0.8).
Interestingly, in this trial the occurrence of AEs was similar in both arms. In both trials docetaxel and the ARPI have been given concomitantly. Of the included patients 77% had high volume and 70% high-risk disease. In an unplanned subgroup analysis the beneficial effect of adding darolutamide vs. placebo for OS was seen in the patients with high-volume (HR 0.69; 0.57-0.82), with high-risk (HR 0.71; 0.58-0.86) and in low-risk disease (HR 0.62; 0.42-0.9), for the small subgroup of patients with low-volume disease the results were suggestive of an OS benefit (HR 0.68; 0.41-1.13) [1191].
Also in ENZAMET, TITAN and ARCHES there were patients who received docetaxel as a part of SOC, thus not all concomitantly, but the percentage of patients receiving docetaxel in these trials was much lower [1125,1126,1172,1177-1179].
There are also SRs and network meta-analysis for systemic triplet therapies and they confirm that the triplets are more effective than ADT and docetaxel alone [1192], in one analysis looking into subgroups statistically significant for patients with high volume disease and de novo disease [1193].
A SR and network MA for the different systemic treatments of mHSPC confirms triplets to be more effective than a doublet of ADT and docetaxel but not necessarily better than ADT plus ARPI. For patients with metachronous low-volume PCa, ARPI doublet therapies were ranked as the potentially most efficacious treatment options and the expected outcomes were not significantly different from those achieved by triplet regimens [1194]. In addition, in a MA of individual patient trial data of patients with metachronous low volume prostate cancer did not benefit from receiving docetaxel [1187].
6.6.5. Treatment selection and patient selection
There have been several network meta-analyses of the published data concluding that combination therapy is more efficient than ADT alone, but none of the doublet combination therapies has been convincingly proven to be superior over another [1194-1199]. In a SR and meta-analysis looking at association between age and efficacy of combination therapy patients seemed to profit from combination therapy irrespective of age [1199]. As a consequence, patients should be offered combination treatment unless there are clear contra-indications or they present with asymptomatic disease and a very short life expectancy (based on frailty assessment or non-cancer co-morbidities).
Since the data of the above mentioned phase III triplet therapy trials have been reported, docetaxel as sole addition to ADT is no longer a valid option in the majority of patients if an androgen receptor pathway inhibitor (ARPI) is available and there are no contra-indications to use one. From subgroup analysis of all the above-mentioned RCTs we know that probably all subgroups (high vs. low volume/risk and synchronous vs. metachronous) can profit from the addition of an ARPI to ADT. Therefore, in view of the current data the recommendation is using ADT plus ARPI as the sole additional therapy or the triplet with an ARPI plus docetaxel. Formally the question what the added value of adding docetaxel to ADT plus an ARPI has not been evaluated. The data should be discussed with patients who are fit for chemotherapy and an ARPI, realising that most of the toxicity is caused by adding the chemotherapy. There is more evidence for using the triplet in synchronous disease and the OS benefit in PEACE-1 seemed to be driven mostly by the high volume patients at the time point of the analysis for the publication, in ARASENS only few patients had low volume disease. A living SR and MA, providing continuously automated updates is recommended for review [1194].
The choice of treatment will most likely be driven by fitness for docetaxel, the nature of the disease (low/high volume; synchronous/metachronous), patient preference, the specific side effects, availability, logistics and cost. The lack of high-level evidence for the benefit of triplet (ADT+ARPI+docetaxel) vs. doublet (ADT+ARPI) makes it difficult to make a strong recommendation for one option over the other including for patients with synchronous high-volume mHSPC.
6.6.6. Treatment of the primary tumour in newly diagnosed metastatic disease
The first reported trial evaluating prostate RT in men with metastatic castration-sensitive disease was the HORRAD trial. Four hundred and thirty-two patients were randomised to ADT alone or ADT plus IMRT with IGRT to the prostate. Overall survival was not significantly different (HR: 0.9 [0.7–1.14]), median time to PSA progression was significantly improved in the RT arm (HR: 0.78 [0.63–0.97]) [1200]. The STAMPEDE trial evaluated 2,061 men with metastatic castration-sensitive PCa (mCSPC) who were randomised to ADT alone vs. ADT plus RT to the prostate. This trial confirmed that RT to the primary tumour did not improve OS in unselected patients [1152]. However, following the results from CHAARTED and prior to analysing the data, the original screening investigations were retrieved, and patients categorised as low- or high volume. In the low-volume subgroup (n = 819) there was a significant OS benefit by the addition of prostate RT. This was confirmed by the latest analysis of long-term follow-up (median follow-up of 61 months [HR: 0.64 for OS benefit in the low-volume group]) [1201].
A secondary, not pre-planned analysis of the STAMPEDE trial confirmed the benefit of prostate RT in patients with ≤ 3 bone metastases, but also showed a benefit in patients with M1a disease [1202]. No evidence of difference in time to symptomatic local events was found with median follow-up of over five years [1201].The dose used in these indications should be equivalent of up to 72 Gy in 2 Gy fractions. Therefore, RT of the prostate only in patients with low-volume metastatic disease should be considered.
A network meta-analysis demonstrates that adding prostate RT to ADT alone results in 27% reduction in the hazard for death (pooled HR: 0.73; 95% credible interval [CrI]: 0.62–0.87), while ADT plus ARPI was associated with a 32% reduction (pooled HR: 0.68; 95% CrI: 0.60–0.78) and ADT plus ARPI plus RT was associated with a 47% reduction (pooled HR: 0.53; 95% CrI: 0.34–0.81) in the hazard for death (in risk of death) [1203]. It is not clear if these data can be extrapolated to RP as local treatment as results of ongoing trials are awaited.
In a SR and meta-analysis including the above two RCTs, the authors found that, overall, there was no evidence that the addition of prostate RT to ADT improved survival in unselected patients (HR: 0.92, 95% CI: 0.81–1.04, p = 0.195) [1204]. However, there was a clear difference in the effect of metastatic burden on survival with an absolute improvement of 7% in three-year survival in men who had four or fewer bone metastases.
The randomised phase-III trial with a 2 × 2 factorial design PEACE-1 (SOC, SOC+Abiraterone, SOC+RT and SOC+Abiraterone+RT) including 1,172 patients demonstrated that adding prostate radiotherapy (total dose 74 Gy in 37 fractions) significantly prolonged the co-primary endpoint of PFS from 4.4 years to 7.5 years in the low-volume metastatic burden group treated with SOC+ARPI. Additionally, a significant delay in the time to castration resistance was observed, although there was no improvement in OS in this group [1205].
PEACE-1 also reported a significant reduction in the incidence of serious genitourinary events such as obstruction, bleeding, insertion of double-J stent and TURP for patients treated with local RT to the prostate. This was an important secondary endpoint in the PEACE-1 study where the preventive effect of radiotherapy was observed both in the cohort of patients with low-volume metastatic disease (26% vs. 11%; delay in the time to first serious genitourinary event p = 0·0002;) and the overall cohort (22.3% vs 12.2%; p = 0·0001) [1205].
6.6.7. Metastasis-directed therapy in M1-patients
In patients relapsing after a local treatment, a metastases-targeting therapy has been proposed, with the aim to delay systemic treatment. In a retrospective analysis on 211 patients treated with MDT, Milenkovic et al. aimed at defining prognostic factors for MFS, palliative ADT-free (pADT) survival and cause-specific survival (CSS). With a median follow-up of 42 months after MDT, patients with cN1 only had significantly superior five-years MFS, pADT and CSS when compared to patients with M1 disease (p<0.02). Of interest, 23% of patients were free of biochemical recurrence at five years [1206]. There are two randomised phase II trials testing metastasis-directed therapy (MDT) using surgery ± SABR vs. surveillance [1207] or SABR vs. surveillance in men with oligo-recurrent PCa [1208]. Oligo-recurrence was defined as < 3 lesions on choline-PET/CT only [1207] or conventional imaging with MRI/CT and/or bone scan [1208]. The sample size was small with 62 and 54 patients, respectively, and a substantial proportion of them had nodal disease only [1207]. Androgen deprivation therapy-free survival was the primary endpoint in one study which was longer with MDT than with surveillance [1207]. The primary endpoint in the ORIOLE trial was progression after six months which was significantly lower with SBRT than with surveillance (19% vs. 61%, p = 0.005) [1208].
Recently the combined results of STOMP and ORIOLE confirmed the significant improvement in PFS in favour of MDT (HR: 0.44, p < 0.001) [1209].
A phase II trial assessed the biochemical response after 18F-DCFPyL PET/MRI and subsequent MDT. Overall biochemical response rate, defined as ≥ 50% PSA decline, was 60%, including 22% of patients with complete biochemical response [1210].
The phase II randomised EXTEND trial investigated whether MDT when added to standard-of-care systemic treatment improved PFS when compared to standard-of-care systemic treatment alone in oligometastatic prostate cancer patients, with oligometastatic being defined as maximally 5 lesions. In total, 87 patients were randomized and the vast majority presented with 1 or 2 metastatic lesions. In total, 51 patients received ADT alone, while 36 patients also received ARPI. The addition of MDT significantly improved both PFS (15.8 months vs. not reached; HR: 0.25; p<0.001) and eugonadal PFS (6.1 months vs. not reached; HR: 0.32; p = 0.03). This significant benefit was observed both in the patient group receiving ADT alone or ADT + ARPI [1211]. In analogy, the SATURN trial, which included 28 oligo-recurrent metastatic prostate cancer patients, looked at the PFS of adding dual ARPI and MDT to existing ADT. The median PFS in SATURN was 19.3 months and 50% of the patients still had an undetectable PSA six months after testosterone recovery. While MDT-induced toxicity was very low, adding dual ARPI induced grade 3 toxicity in 20% of the patients [1212].
Currently there are no data to suggest an improvement in OS. Two comprehensive reviews highlighted MDT (SABR) as a promising therapeutic approach that must still be considered as investigational until the results of the ongoing RCT are available [1213,1214]. The toxicity of MDT is low, with nearly no grade ≥ 3 toxicity [1215-1217].
6.6.8. Recommendations for the first-line treatment of hormone-sensitive metastatic disease*
| Recommendations | Strength rating |
| First-line treatment | |
| Discuss all patients with hormone-sensitive metastatic disease in a multidisciplinary team. | Strong |
| Offer immediate systemic treatment with androgen deprivation therapy (ADT) to palliate symptoms and reduce the risk for potentially serious sequelae of advanced disease (spinal cord compression, pathological fractures, ureteral obstruction) to M1 symptomatic patients. | Strong |
| Offer short-term administration of an older generation androgen receptor (AR) antagonist to M1 patients starting luteinising hormone-releasing hormone (LHRH) agonist to reduce the risk of the ‘flare-up’ phenomenon. | Weak |
| At the start of ADT offer LHRH antagonists or orchiectomy to patients with impending clinical complications such as spinal cord compression or bladder outlet obstruction. | Strong |
| Do not offer AR antagonist monotherapy to patients with M1 disease. | Strong |
| Do not offer ADT monotherapy to patients whose first presentation is M1 disease if they have no contra-indications for combination therapy and have a sufficient life expectancy to benefit from combination therapy (≥ 1 year) and are willing to accept the increased risk of side effects. | Strong |
| Offer ADT combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide to patients with M1 disease who are fit for the regimen. | Strong |
| Offer ADT combined with darolutamide to patients with M1 disease who are fit for the regimen. | Weak |
| Offer docetaxel only in combination with ADT plus abiraterone or darolutamide to patients with M1 disease who are fit for docetaxel. | Strong |
| Offer ADT combined with prostate radiotherapy (using doses up to the equivalent of 72 Gy in 2 Gy fractions) to patients whose first presentation is M1 disease and who have low volume of disease by CHAARTED criteria. | Strong |
| Do not offer ADT combined with surgery to M1 patients outside of clinical trials. | Strong |
| Only offer metastasis-directed therapy to M1 patients within a clinical trial setting or a well-designed prospective cohort study. | Strong |
| Supportive care | |
| Assess osteoporosis risk factors and perform a dexa scan when commencing long-term ADT, to mitigate osseous complications. | Strong |
| Offer bone protection to avoid fractures in patients receiving combination treatment. | Strong |
| Offer calcium and vitamin D supplementation when prescribing either denosumab or bisphosphonates and monitor serum calcium. | Strong |
| Treat painful bone metastases early on with palliative measures such as intensity-modulated radiation therapy/volumetric arc radiation therapy plus image-guided radiation therapy and adequate use of analgesics. | Strong |
| In patients with spinal cord compression start immediate high-dose cortico-steroids and assess for spinal surgery potentially followed by radiation. Offer radiation therapy alone if surgery is not appropriate. | Strong |
*All the following statements are based on metastatic disease defined by bone scintigraphy and CT scan/MRI.
6.7. Treatment: Castration-resistant PCa (CRPC)
6.7.1. Definition of CRPC
Castrate serum testosterone < 50 ng/dL or 1.7 nmol/L plus either:
- Biochemical progression: Three consecutive rises in PSA at least one week apart resulting in two 50% increases over the nadir, and a PSA > 2 ng/mL; or
- Radiological progression: The appearance of new lesions: either two or more new bone lesions on bone scan, ideally confirmed [1218], or a soft tissue lesion using RECIST (Response Evaluation Criteria in Solid Tumours) [1219]. Symptomatic progression alone must be questioned and subject to further investigation. It is not sufficient to diagnose CRPC.
- Unequivocal clinical progression.
6.7.2. Management of mCRPC - general aspects
Selection of treatment for mCRPC is multifactorial and in general dependent on:
- previous treatment for mHSPC and for non-mHSPC;
- previous treatment for nmCRPC and mCRPC;
- quality of response and pace of progression on previous treatment;
- known cross resistance between androgen receptor pathway inhibitor (ARPI);
- co-medication and known drug interactions (see approved summary of product characteristics);
- known genetic alterations and microsatellite instability–high (MSI-H)/mismatch repair-deficient (dMMR) status;
- known histological variants and DNA repair deficiency (to consider platinum or targeted therapy like PARPi);
- local approval status of drugs and reimbursement situation;
- available clinical trials;
- the patient and his co-morbidities.
6.7.2.1. Molecular diagnostics
All metastatic patients should be offered somatic genomic testing for homologous repair and MMR defects early on, preferably before first-line mCRPC treatment is established. Testing should preferably be performed on metastatic carcinoma tissue but testing on primary tumour may also be performed. Alternatively, but still less common, genetic testing on circulating tumour DNA (ctDNA) is an option and has been used in some trials. One test, the FoundationOne® Liquid CDx, has been FDA approved [1220]. Defective MMR assessment can be performed by IHC for MMR proteins (MSH2, MSH6, MLH1 and PMS2) and/or by next generation sequencing (NGS) assays [1221]. Germline testing for BRCA1/2, ATM and MMR is recommended for high-risk- and particularly for metastatic PCa if clinically indicated.
Molecular diagnostics should be performed by a certified (accredited) institution using a standard NGS multiplication procedure (minimum depth of coverage of 200 X). The genes and respective exons should be listed; not only DNA for mutations but RNA needs to be examined for fusions and protein expression to obtain all clinically relevant information. A critical asset is the decision support helping to rate the mutations according to their clinical relevance [1222,1223]. Ideally, a molecular tumour board is involved to support interpretation of the report and clinical decision taking.
Level 1 evidence for the use of PARP-inhibitors has been reported [273,1224-1235]. Microsatellite instability (MSI)-high (or MMR deficiency) is rare in PCa, but for those patients, pembrolizumab has been approved by the FDA and could be a valuable additional treatment option [1141,1236]. Germline molecular testing is discussed in section 5.1.7 and recommendations for germline testing are provided in section 5.1.8.
6.7.3. Treatment decisions and sequence of available options
Approved agents for the treatment of mCRPC in Europe are docetaxel, abiraterone/prednisolone (AAP), enzalutamide, cabazitaxel, olaparib, niraparib/AAP, talazoparib/enzalutamide, radium-223 and lutetium (177Lu) vipivotide tetraxetan. Regarding CRPC, darolutamide and apalutamide have been approved only for nmCRPC. In general, sequencing of ARPIs like abiraterone and enzalutamide is not recommended particularly if the time of response to ADT and to the first ARPI was short (≤ six to twelve months) and high-risk features of rapid progression are present (see detailed discussion in section 6.7.7) [1237-1239].
The use of chemotherapy with docetaxel and subsequent cabazitaxel in the treatment sequence is recommended and should be applied early enough when the patient is still fit for chemotherapy. This is supported by high-level evidence [1237].
In case of a known BRCA alteration, the use of a PARP inhibitor should always be prioritised as its use improves rPFS and OS [1240-1243].
6.7.4. Non-metastatic CRPC
Frequent PSA testing in men treated with ADT has resulted in earlier detection of biochemical progression. Of these men approximately one-third will develop bone metastases within two years, detected by conventional imaging [952].
In men with CRPC and no detectable clinical metastases using bone scan and CT-scan, baseline PSA level, PSA velocity and PSA-DT have been associated with time to first bone metastasis, bone MFS and OS [952,1244]. These factors may be used when deciding which patients should be evaluated for metastatic disease. A consensus statement by the PCa Radiographic Assessments for Detection of Advanced Recurrence (RADAR) group suggested a bone scan and a CT scan when the PSA reached 2 ng/mL and if this was negative, it should be repeated when the PSA reached 5 ng/mL, and again after every doubling of the PSA based on PSA testing every three months in asymptomatic men [1245]. Symptomatic patients should undergo relevant investigations regardless of PSA level. With more sensitive imaging techniques like PSMA PET/CT or whole-body MRI, more patients are diagnosed with early mCRPC [1246]. It remains unclear if the use of PSMA PET/CT in this setting improves outcome.
Three large phase III RCTs, PROSPER [1247], SPARTAN [1248] and ARAMIS [1249], evaluated MFS as the primary endpoint in patients with nmCRPC (M0 CRPC) treated with enzalutamide (PROSPER) vs. placebo or apalutamide (SPARTAN) vs. placebo or darolutamide (ARAMIS) vs. placebo, respectively (Table 6.7.1). The M0 status was established by CT and bone scans. Only patients at high risk for the development of metastasis with a short PSA-DT of ≤ ten months were included. Patient characteristics in the trials revealed that about two-thirds of participants had a PSA-DT of < 6 months. All trials showed a significant MFS benefit. All three trials showed a survival benefit after a follow-up of more than 30 months. In view of the long-term treatment with these AR targeting agents in asymptomatic patients, potential AEs need to be taken into consideration and the patient informed accordingly.
6.7.5. Metastatic CRPC
The remainder of this section focuses on the management of men with proven mCRPC on conventional imaging.
6.7.5.1. Conventional androgen deprivation in CRPC
Eventually men with PCa will show evidence of disease progression despite castration. Two trials have shown only a marginal survival benefit for patients remaining on LHRH analogues during second- and thirdline therapies [1250,1251]. However, in the absence of prospective data, the modest potential benefits of continuing castration outweigh the minimal risk of treatment. In addition, all subsequent treatments have been studied in men with ongoing androgen suppression, therefore, it should be continued in these patients.
6.7.6. First-line treatment of metastatic CRPC
6.7.6.1. Abiraterone
Abiraterone was evaluated in 1,088 chemo-naive, asymptomatic or mildly symptomatic mCRPC patients in the phase III COU-AA-302 trial. Patients were randomised to abiraterone acetate or placebo, both combined with prednisone [1252]. Patients with visceral metastases were excluded. The main stratification factors were ECOG PS 0 or 1 and asymptomatic or mildly symptomatic disease. Overall survival and rPFS were the co-primary endpoints. After a median follow-up of 22.2 months there was significant improvement of rPFS (median 16.5 vs. 8.2 months, HR: 0.52, p < 0.001) and the trial was unblinded. At the final analysis with a median follow-up of 49.2 months, the OS endpoint was significantly positive (34.7 vs. 30.3 months, HR: 0.81, 95% CI: 0.70–0.93, p = 0.0033) [1253]. Adverse events related to mineralocorticoid excess and liver function abnormalities were more frequent with abiraterone, but mostly grade 1–2. Subset analysis of this trial showed the drug to be equally effective in an elderly population (> 75 years) [1254].
6.7.6.2. Enzalutamide
A randomised phase III trial (PREVAIL) included a similar patient population and compared enzalutamide and placebo [1255]. Men with visceral metastases were eligible but the numbers included were small. Corticosteroids were allowed but not mandatory. PREVAIL was conducted in a chemo-naive mCRPC population of 1,717 men and showed a significant improvement in both co-primary endpoints, rPFS (HR: 0.186, CI: 0.15–0.23, p < 0.0001), and OS (HR: 0.706, CI: 0.6–0.84, p < 0.001). A ≥ 50% decrease in PSA was seen in 78% of patients. The most common clinically relevant AEs were fatigue and hypertension. Enzalutamide was equally effective and well tolerated in men > 75 years [1256] as well as in those with or without visceral metastases [1257]. However, for men with liver metastases, there seemed to be no discernible benefit [1257,1258].
Enzalutamide has also been compared with bicalutamide 50 mg/day in a randomised double-blind phase II study (TERRAIN) showing a significant improvement in PFS (15.7 months vs. 5.8 months, HR: 0.44, p < 0.0001) in favour of enzalutamide [1258]. With extended follow-up and final analysis the benefit in OS and rPFS were confirmed [1259].
6.7.6.3. Docetaxel
A statistically significant improvement in median survival of 2.0–2.9 months has been shown with docetaxel compared to mitoxantrone plus prednisone [1260,1261]. The standard first-line chemotherapy is docetaxel 75 mg/m2, 3-weekly doses combined with prednisone 5 mg twice a day (BID), up to ten cycles. Prednisone can be omitted if there are contra-indications or no major symptoms. The following independent prognostic factors: visceral metastases, pain, anaemia (Hb < 13 g/dL), bone scan progression, and prior estramustine may help stratify the response to docetaxel. Patients can be categorised into three risk groups: low risk (0 or 1 factor), intermediate (2 factors) and high risk (3 or 4 factors), and show three significantly different median OS estimates of 25.7, 18.7 and 12.8 months, respectively [1262].
Age by itself is not a contra-indication to docetaxel [1263] but attention must be paid to careful monitoring and co-morbidities as discussed in section 6.1 - Estimating life expectancy and health status [1264]. In men with mCRPC who are thought to be unable to tolerate the standard dose and schedule, docetaxel 50 mg/m2 every two weeks seems to be well tolerated with less grade 3–4 AEs and a prolonged time to treatment failure [1265].
6.7.6.4. Sipuleucel-T
In 2010 a phase III trial of sipuleucel-T showed a survival benefit in 512 asymptomatic or minimally symptomatic mCRPC patients [1266]. After a median follow-up of 34 months, the median survival was 25.8 months in the sipuleucel-T group compared to 21.7 months in the placebo group, with a HR of 0.78 (p = 0.03). No PSA decline was observed and PFS was similar in both arms. The overall tolerance was very good, with more cytokine-related AEs grade 1–2 in the sipuleucel-T group, but the same grade 3–4 AEs in both arms. Sipuleucel-T is not available in Europe.
6.7.6.5. Combinations with PARP inhibitors
Based on the suggestion that there is a synergistic antitumour effect when combining an ARPI with a PARP inhibitor, several such combination trials were conducted in first-line mCRPC patients with different trial designs, different patient selection and conflicting results.
Abiraterone/prednisone plus olaparib
A randomised double-blind, phase III trial (PROpel) of AAP plus olaparib (300 mg twice daily) or placebo in patients with mCRPC in the first-line setting was conducted [1226,1227]. Patients (n = 796) were randomly assigned 1:1 to study treatment regardless of homologous recombination repair gene mutation (HRRm) status which was retrospectively evaluated and determined by tumour tissue and circulating tumour DNA tests. The primary end point was imaging-based PFS (ibPFS) by investigator assessment. The result was significantly positive in favour of the combination with ibPFS of 24.8 vs. 16.6 mo (HR 0.66; 95% CI: 0.54 to 0.81; p = 0.001). In the prespecified final analyses the key secondary endpoint OS had only 47.9% maturity and did not meet the prespecified 2-sided boundary for significance (HR 0.95% CI: 0.81, 0.67-1.0, p = 0.054). The subgroup of patients with positive HRRm status showed a rPFS HR of 0.50 (CI: 0.34 to 0.73). The BRCA mutated patients (11% of the ITT population) had an even larger benefit for rPFS (HR 0.24; 95% CI: 0.12, 0.45) and the OS HR in these patients was 0.30 (95% CI: 0.15, 0.59), suggesting that the improvement in rPFS observed in the ITT population was primarily driven by patients with a BRCA mutation [1228].
The most common AEs in patients receiving olaparib plus AAP were anaemia (48%; ≥ G3 15%), fatigue (38%), nausea (30%), diarrhoea (19%), decreased appetite (16%), lymphopenia (14%), dizziness (14%), and abdominal pain (13%); 18% of patients required at least one blood transfusion and 12% required multiple transfusions [1228]. The combination of olaparib plus AAP was approved by the EMA for the treatment of adult patients with mCRPC in whom chemotherapy is not clinically indicated [1240]. In the US, the FDA has approved olaparib with AAP for mCRPC patients with deleterious or suspected deleterious BRCA-mutations as determined by an FDA-approved companion diagnostic test [1229]. For patients without BRCAm, the FDA determined that the modest rPFS improvement, combined with clinically significant toxicities, did not demonstrate a favorable risk/benefit assessment [1241].
The combination of PARP inhibitor plus ARPI in patients with BRCA1/2 (or ATM) mutations in the first-line as opposed to the use of PARP inhibitor monotherapy or the sequential use of these agents is supported by a randomised phase II trial albeit with low patient numbers and thus a low level of evidence [1267].
Abiraterone/prednisone plus niraparib
In a randomised, double-blind, phase III trial (MAGNITUDE) AAP plus niraparib 200 mg once/daily or placebo, was evaluated [1230]. The study prospectively included 2 cohorts, an HRR-negative and an HRR-positive cohort. The HRR-negative cohort was closed early for futility after enrolling 200 patients. In the overall HRR-positive cohort, the addition of Niraparib to AAP resulted in a significant improvement in the first endpoint rPFS compared to AAP plus placebo (HR = 0.73; 95% CI 0.56-0.96; p = 0.0217) and the median rPFS was 16.5 vs. 13.7 months in favour of the combination. In particular, the 113 patients with BRCA 1/ 2 mutations [1231] who received AAP plus niraparib [1231] derived a major rPFS benefit (19.5 vs. 10.9 months; HR = 0.55 [95% CI 0.39-0.78]; nominal p = 0.0007). The OS data is still immature. The most common side effects with Niraparib plus AAP in the ITT population were anemia (46.2%), fatigue (26.4%), hypertension (31.6%) and constipation (30.7%). The combination of niraparib plus AAP in a dual-action tablet has been approved by the EMA and the FDA for patients with mCRPC and BRCA 1/2 mutations in whom chemotherapy is not clinically indicated [1232].
Enzalutamide plus Talazoparib
A randomised double-blind, phase III trial (TALAPRO-2) of the PARP inhibitor talazoparib (0.5mg daily) plus enzalutamide versus enzalutamide/placebo showed a significantly better median rPFS (first endpoint) in favour of the combintion regardless of the HRR pathway status [1233].
The median rPFS was not yet reached for the combination as compared to 21.9 mo in the control arm (95% CI 16.6-25.1). The HR for rPFS was 0.63 (0.51-0.78) with p<0.0001. For the subgroups of patients with HRR mutations the benefit of the combination was much more pronounced. The HRR gene-mutated population showed a median rPFS of 27.9 (16.6–not reached) for the talazoparib combination versus 16.4 (10.9–24.6) for the placebo group (0.46; 95% CI: 0.30–0.70; p = 0.0003 ) and 0.70 (0.54–0.89; p = 0.0039) in patients with a status of non-deficient or unknown. In an exploratory analysis, the HR for rPFS in patients with BRCA-mutated mCRPC was 0.23 (0.10–0.53; p = 0.0002) and, in patients with non-BRCAm HRR gene-mutated mCRPC, it was 0.66 (0.39–1.12; p = 0.12) in favour of the talazoparib combination [1233]. The OS data were still immature. The expected clinical benefit in the subgroups needs to be weighed against the potential burden of side effects [1240].
The most common treatment-emergent AEs with the addition of talazoparib were anaemia, neutropenia, and fatigue; the most common grade 3–4 event was anaemia (46%), which improved after dose reduction, however, 39% required a blood transfusion, including 22% who required multiple transfusions, 8% discontinued treatment due to anaemia and two patients on the combination were diagnosed with myelodysplastic syndrome/acute myeloid leukaemia [1233]. In TALAPRO-2 an HRR-deficient-only cohort (cohort 2; n = 230) was recruited. The primary analysis for the combined HRR-deficient population (n = 399) met the rPFS endpoint with a HR 0.45 (95% CI, 0.33 to 0.61; p < 0.0001; median not reached at the time of the analysis for the talazoparib group versus 13.8 months for the placebo group). Also for this cohort data for OS were immature but favour talazoparib (HR 0.69; 95% confidence interval, 0.46 to 1.03; p = 0.07) [1234].
The FDA approved talazoparib with enzalutamide only for HRR gene-mutated mCRPC [1235,1240,1268]. The EMA has approved the combination of talazoparib and enzalutamide for the treatment of patients with mCRPC in whom chemotherapy is not clinically indicated [1269].
Regarding additional side effects of special interest, there seems to be a doubling of the risk of thromboembolic events with the use of PARPis. In a meta-analysis of 2,210 and 1,662 patients with PC and PARPi treatment vs. control, PARPi had a statistically significant increased risk of thrombosis in PCa patients (OR = 1.98, 95 % CI: 1.06–3.70, p = 0.030) with 96 (4.3 %) and 37 (2.2 %) in the PARPi and control groups, respectively [1270].
Based on eighteen placebo-controlled RCTs (n = 7,307 patients, tumour agnostic), PARPis significantly increased the risk of myelodysplastic syndrome and acute myeloid leukaemia compared with placebo treatment (Peto OR 2·63 [95% CI 1·13–6·14], p = 0·026) with no between-study heterogeneity (I2=0%, χ2 p = 0·91). Median treatment duration was 9.8 months (IQR 3·6–17·4; n = 96) and median latency period since first exposure to a PARPi was 17.8 months (8·4–29·2; n = 58). Of 104 cases that reported outcomes, 47 (45%) resulted in death [1271].
6.7.7. Second-line treatment for mCRPC
All patients who receive treatment for mCRPC will eventually progress. All treatment options in this setting are presented in Table 6.7.3. There is a paucity of high-level data with regards to the sequence of treatments in case of pretreatment with ARPI and/or docetaxel for mHSPC.
6.7.7.1. Cabazitaxel
Cabazitaxel is a taxane with activity in docetaxel-resistant cancers. It was studied in a large prospective, randomised, phase III trial (TROPIC) comparing cabazitaxel plus prednisone vs. mitoxantrone plus prednisone in 755 patients with mCRPC, who had progressed after or during docetaxel-based chemotherapy [1272]. Patients received a maximum of ten cycles of cabazitaxel (25 mg/m2) or mitoxantrone (12 mg/m2) plus prednisone (10 mg/day). Overall survival was the primary endpoint which was significantly longer with cabazitaxel (median: 15.1 vs. 12.7 months, p < 0.0001). There was also a significant improvement in PFS (median: 2.8 vs. 1.4 months, p < 0.0001), objective RECIST response (14.4% vs. 4.4%, p < 0.005), and PSA response rate (39.2% vs. 17.8%, p < 0.0002). Treatment-associated WHO grade 3–4 AEs developed significantly more often in the cabazitaxel arm, particularly haematological (68.2% vs. 47.3%, p < 0.0002) but also non-haematological (57.4 vs. 39.8%, p < 0.0002) toxicity. In two post-marketing randomised phase III trials, cabazitaxel was shown not to be superior to docetaxel in the first-line setting; in the second-line setting in terms of OS, 20 mg/m2 cabazitaxel was not inferior to 25 mg/m2, but less toxic. Therefore, the lower dose should be preferred [1273,1274]. Cabazitaxel should preferably be given with prophylactic granulocyte colonystimulating factor (G-CSF) and should be administered by physicians with expertise in handling neutropenia and sepsis [1275].
6.7.7.2. Abiraterone acetate after docetaxel for mCRPC
Positive results of the large phase III trial (COU-AA-301) were reported after a median follow-up of 12.8 months [1276] and confirmed by the final analysis [1277]. A total of 1,195 patients with mCRPC were randomised 2:1 to AAP or placebo plus prednisone. All patients had progressive disease based on the Prostate Cancer Clinical Trials Working Group 2 (PCWG2) criteria after docetaxel therapy (with a maximum of two previous chemotherapeutic regimens). The primary endpoint was OS, with a planned HR of 0.8 in favour of AAP. After a median follow-up of 20.2 months, the median survival in the AAP group was 15.8 months compared to 11.2 months in the placebo arm (HR: 0.74, p < 0.0001). The benefit was observed in all subgroups and all the secondary objectives were in favour of AAP (PSA, radiologic tissue response, time to PSA or objective progression). The incidence of the most common grade 3–4 AEs did not differ significantly between arms, but mineralocorticoid-related side effects were more frequent in the AAP group, mainly grade 1–2 (fluid retention, oedema and hypokalaemia).
6.7.7.3. Enzalutamide after docetaxel for mCRPC
The planned interim analysis of the AFFIRM study was published in 2012 [1278]. This trial randomised 1,199 patients with mCRPC in a 2:1 fashion to enzalutamide or placebo. The patients had progressed after docetaxel treatment, according to the PCWG2 criteria. Corticosteroids were not mandatory, but could be prescribed, and were received by about 30% of the patients. The primary endpoint was OS, with an expected HR benefit of 0.76 in favour of enzalutamide. After a median follow-up of 14.4 months, the median survival in the enzalutamide group was 18.4 months compared to 13.6 months in the placebo arm (HR: 0.63, p < 0.001). This led to the recommendation to halt and unblind the study. The benefit was observed irrespective of age, baseline pain intensity, and type of progression. In the final analysis with longer follow-up the OS results were confirmed despite crossover and extensive post-progression therapies [1223]. Enzalutamide was active also in patients with visceral metastases.
All the secondary objectives were in favour of enzalutamide (PSA, soft tissue response, QoL, time to PSA, or objective progression). No difference in terms of side effects was observed in the two groups, with a lower incidence of grade 3–4 AEs in the enzalutamide arm. There was a 0.6% incidence of seizures in the enzalutamide group compared to none in the placebo arm.
6.7.7.4. Radium-223 after ARPI or both ARPI and docetaxel for mCRPC
The only bone-specific drug that is associated with a survival benefit is the α-emitter radium-223. In a large phase III trial (ALSYMPCA) 921 patients with symptomatic mCRPC, who failed or were unfit for docetaxel, were randomised to six injections of 50 kBq/kg radium-223 or placebo plus SOC. The primary endpoint was OS. Radium-223 significantly improved median OS by 3.6 months (HR: 0.70, p < 0.001) and was also associated with prolonged time to first skeletal event, improvement in pain scores and improvement in QoL [1279]. The associated toxicity was mild and, apart from slightly more haematologic toxicity and diarrhoea with radium-223, did not differ significantly from that in the placebo arm [1279]. Radium-223 was effective and safe whether or not patients were docetaxel pre-treated [1280]. Due to safety concerns, use of radium-223 was restricted to after docetaxel and at least one AR targeted agent [1281]. In particular, the use of radium-223 in combination with AAP showed significant safety risks related to fractures and more deaths. This was most striking in patients without the concurrent use of bone health agents [1282] so that radium-223 should always be used together with bone health agents (see section 6.7.11.2)
6.7.7.5. Rucaparib after ARPI 1243
In a 2:1 randomised, controlled, phase III trial (TRITON-3) 405 mCRPC patients were included. Patients were selected for a BRCA1, BRCA2, or ATM alteration and disease progression after treatment with an ARPI for mCRPC. Treatment was as follows: rucaparib 600 mg twice daily or a physician’s choice control, either second line docetaxel or the ARPI which had not been given previously. The first endpoint rPFS in the intention-to-treat group was significantly better with rucaparib (median, 10.2 months and 6.4 months, respectively; HR 0.61; 95% CI, 0.47 to 0.80; p < 0.001). The small ATM subgroup did not derive a benefit. An interim analysis revealed OS to be immature. The study design allowed for cross-over and 60% of patients received a PARP inhibitor at progression (47% rucaparib). With regards to the control arms, the median rPFS was longer with rucaparib than with docetaxel (11.2 months vs. 8.3 months; hazard ratio, 0.53; 95% CI, 0.37 to 0.77) and it was also longer than with an ARPI (11.2 months vs. 4.5 months; hazard ratio, 0.38; 95% CI, 0.25 to 0.58). The most frequent AEs with rucaparib were fatigue, nausea and anaemia, including 24% Grade ≥ 3 anaemia and 29% of patients on rucaparib required at least one blood transfusion [1283]. Rucaparib has been approved by the FDA.
6.7.7.6. Olaparib after ARPI
See section 6.7.8.3 PARP inhibitors for mCRPC.
6.7.7.7. 177Lu-PSMA-617 after ARPI
Primary and updated analyses of rPFS for the phase III, multicenter RCT, PSMAfore, investigating taxane-naive patients with PSMA-positive mCRPC who had progressed on ARPI, have been published. Patients were 1:1 randomised between open-label, intravenous ¹⁷⁷Lu-PSMA-617 (7.4 GBq intravenously, every 6 weeks, for up to 6 cycles) and a change of ARPI. A total of 468 patients met all eligibility criteria and were randomly assigned to receive ¹⁷⁷Lu-PSMA-617 (234 [50%] patients) or ARPI change (234 [50%]). Of the 234 patients assigned to ARPI change, 134 (57%) crossed over to receive 177Lu-PSMA-617. In the updated analysis at time of the third data cut-off (median time from randomisation to third data cut-off 24.11 months [IQR 20.24–27.40]), median rPFS was 11.60 months (95% CI: 9.30–14.19) in the ¹⁷⁷Lu-PSMA-617 group vs. 5.59 months (4.21–5.95) in the ARPI change group (HR 0.49 [95% CI: 0.39–0.61]). The incidence of grade 3–5 AEs was lower in the ¹⁷⁷Lu-PSMA-617 group compared to the ARPI change group. The key secondary endpoint OS was similar in both groups [1284].
6.7.8. Treatment after docetaxel and one line of hormonal treatment for mCRPC
6.7.8.1. General considerations
For men progressing quickly on AR targeted therapy (< 12 months) it is now clear that cabazitaxel is the treatment supported by the best data. The CARD trial, an open label randomised phase III trial, evaluated cabazitaxel after docetaxel and one line of ARPI (either AAP or enzalutamide) [1237]. It included patients progressing in less than twelve months on previous abiraterone or enzalutamide for mCRPC. Cabazitaxel more than doubled rPFS vs. another ARPI and reduced the risk of death by 36% vs. ARPI. The rPFS with cabazitaxel remained superior regardless of the ARPI sequence and if docetaxel was given before, or after, the first ARPI.
The choice of further treatment after docetaxel and one line of HT for mCRPC is open for patients who have a > twelve months response to first-line abiraterone or enzalutamide for mCRPC [1259]. Either second-line chemotherapy (cabazitaxel), radium-223 (if bone-only metastases), 177Lu–PSMA-617 radioligand therapy [1285,1286] and PARP inhibitors (if BRCA mutation) are valuable options.
Men previously treated with at least one ARPI or both an ARPI and docetaxel and whose tumours demonstrated homozygous deletions or deleterious mutations in DNA-repair genes showed an 88% response rate to olaparib [1287] and in another confirmatory trial a composite response of 54.3% (95% CI: 39.0–69.1) in the 400 mg cohort and in 18 of 46 (39.1%; 25.1–54.6) evaluable patients in the 300 mg cohort [1288]. See also section ‘Second-line management’). In general, subsequent treatments in unselected patients are expected to have less benefit than with earlier use [1289,1290] and there is evidence of cross-resistance between enzalutamide and abiraterone [1291,1292].
6.7.8.2. Radiopharmaceuticals
6.7.8.2.1. Introduction
Historically, several radiopharmaceuticals including phosphorous-32, strontium-89, yttrium-90, samarium-153, and rhenium-186 were developed for the treatment of bone pain secondary to metastases from PCa [1293]. They proved effective in a palliation setting, by relieving pain and improving QoL, especially in the setting of diffuse bone metastases. However, they never gained widespread adoption. The first radioisotope to demonstrate a survival benefit was radium-223 (see Section 6.7.7.4).
6.7.8.2.2. PSMA-based therapy
The increasing use of PSMA PET as a diagnostic tracer and the realisation that this allowed identification of a greater number of metastatic deposits led to attempts to treat cancer by replacing the imaging isotope with a therapeutic isotope which accumulates where the tumour is demonstrated (theranostics) [1294]. Therefore, after identification of the target, usually with diagnostic 68Gallium-labelled PSMA, therapeutic radiopharmaceuticals labelled with β(lutetium-177 or yttrium-90) or α(actinium-225)-emitting isotopes could be used to treat metastatic PCa.
The PSMA therapeutic radiopharmaceutical supported by the most robust data is 177Lu-PSMA-617. The first patient was treated in 2014 and early clinical studies evaluating the safety and efficacy of 177Lu-PSMA therapy have demonstrated promising results, despite the fact that a significant proportion of men had already progressed on multiple therapies [1295]. The early data were based on single-centre experience [1296]. Data from uncontrolled prospective phase II trials reported high response rates with low toxic effects [1297,1298]. Positive signals are also coming from a randomised phase II trial (TheraP) [1299].
In TheraP patients for whom cabazitaxel was considered the next appropriate standard treatment after docetaxel and who were highly selected by 68Ga-PSMA-11 and 18FDG PET-CT scans, were randomised to receive 177Lu-PSMA-617 (6.0–8.5 GBq intravenously, every 6 weeks, for up to 6 cycles) or cabazitaxel (20 mg/m2 for up to ten cycles). The primary endpoint was a reduction of at least 50% in PSA. The first endpoint was met (66% vs. 37% for 177Lu–PSMA-617 vs. cabazitaxel, respectively, by intention to treat; difference 29% (95% CI: 16–42; p < 0.0001; and 66% vs. 44% by treatment received; difference 23% [9–37]; p = 0.0016) [1299]. Secondary outcomes of the TheraP trial, including survival after a median follow-up of 35.7 months (IQR 31.1 to 39.2) showed that 77 (78%) participants had died in the 177Lu-PSMA-617 group and 70 (69%) participants in the cabazitaxel group. Overall survival was similar between randomly assigned patients in the two groups (19.1 vs. 19.6 months; difference -0.5, 95% CI: -3.7 to 2.7]; p = 0.77) [1300,1301].
An open-label phase III trial (VISION) compared 177Lutetium Vipivotid tetraxetan (177Lu-PSMA-617 radioligand therapy) with protocol-permitted SOC (i.e., excluded chemotherapy, immunotherapy, radium-223 and investigational drugs) in mCRPC patients, with PSMA expressing metastases on PET/CT, previously treated with at least one ARPI and one (around 53%) or two taxanes. Imaging-based PFS and OS were the alternate primary endpoints. More than 800 patients were randomised. 177Lu-PSMA-617 plus SOC significantly prolonged both imaging-based PFS and OS, as compared with SOC alone (see Table 6.6.3). Grade 3 or above AEs were higher with 177Lu-PSMA-617 than without (52.7% vs. 38.0%), but QoL was not adversely affected. 177Lu–PSMA-617 has shown to be an additional treatment option in this mCRPC population [1302].
A SR and updated meta-analysis, investigated the proportion of patients with any or more than 50% PSA decrease, and OS. The review, including 69 articles and a total of 4,157 patients, showed that patients treated with 177Lu–PSMA 617 had a significantly higher response to therapy compared to controls, based on ≥ 50% PSA decrease (OR = 5.33, 95% CI: 1.24–22.90, p < 0.05). Meta-analysis revealed an OS of 0.26 according to pooled HRs for any PSA decline, which was significant after 177Lu–PSMA-617 therapy (95% CI: 0.18–0.37, p < 0.00001) and an OS of 0.52 for ≥ 50% PSA decrease, also significant after radioligand (RLT) (95% CI: 0.40–0.67,
p < 0.00001) [1303].
The earlier use of ¹⁷⁷Lu-PSMA-617 was studied in patients progressing on the first ARPI for mCRPC (PSMAfore), see section 6.7.7.7.
There is an increasing interest for PSMA-targeted alpha therapy (225Ac-PSMA) due to the ability to deliver potent higher local radiation more selectively to cancer cells than PSMA-targeted beta therapy, while minimising unwanted damage to the surrounding normal tissues. Additionally, the intensive radiation to cancer cells results in more effective DNA strand breakage and reduces the development of treatment resistance. A meta-analysis, including nine studies with 263 patients, investigated the therapeutic effects of 225Ac-PSMA RLT in patients with metastatic CRPC, pre-treated with chemotherapy, 177Lu-PSMA and/or radium-223. The pooled proportions of patients with more than 50% PSA decline and any PSA decline were 60.99% (95% CI: 54.92%– 66.83%) and 83.57% (95% CI: 78.62%–87.77%), respectively. The estimated mean PFS and mean OS were 9.15 months (95% CI: 6.69–11.03 months) and 11.77 months (95% CI: 9.51–13.49 months), respectively. These findings suggest that 225Ac-PSMA RLT may be an effective treatment option for patients with mCRPC [1304]. Despite the encouraging therapeutic response and survival of patients who received 225Ac-PSMA RLT, major AEs like xerostomia and severe haematotoxicity have to be considered as possible reasons for dose reduction or discontinuation of the therapy.
A retrospective, multicentre international study, WARMTH Act, pooled data of 488 men with mCRPC, who received one or more cycles of 8 MBq 225Ac-PSMA RLT, across 7 international centres [1305]. Patients were heavily pretreated (docetaxel 66%, cabazitaxel 21%, abiraterone 39%, enzalutamide 39%, ¹⁷⁷Lu-PSMA RLT 32%, and 223Ra-dichloride 4%). The median follow-up was 9.0 months. The median OS was 15.5 months (95% CI: 13.4–18.3) and median PFS was 7.9 months (CI: 6.8–8.9). In 347 (71%) out of 488 patients, information regarding treatment-induced xerostomia was available, with 236 (68%) of the 347 patients reporting xerostomia after the first cycle of 225Ac-PSMA RLT. Grade 3 or higher anaemia occurred in 64 (13%) of 488 patients, leukopenia in 19 (4%), thrombocytopenia in 32 (7%), and renal toxicity in 22 (5%). No serious AEs or treatment-related deaths were recorded. This study supports previous data showing that 225Ac-PSMA RLT has a substantial antitumour effect, being a viable therapy option in heavily pretreated mCRPC patients, including patients after ¹⁷⁷Lu-PSMA RLT. Comparable results, with a median OS of 15 months (95% CI: 10-19) for a median follow-up was of 22 months, were reported in a series of patients with mCRPC treated with 225Ac-PSMA (100-150 kBq/kg at least 2 cycles, at 8 weeks), after becoming resistant to all previous anti-cancer agents [1306]. The side effect profile remains to be elucidated. So far, 225Ac-PSMA RLT for mCRPC has not been approved.
Combined therapies, including 177Lu-PSMA-RLT, in mCRPC have moved into the focus of clinical research. In an open-label, multicentre, randomised, phase II trial, EnzaP, participants were randomly assigned (1:1) between getting oral enzalutamide 160 mg daily alone or with adaptive-dosed (two or four doses) 7.5 GBq ¹⁷⁷Lu-PSMA-617 intravenous, every 6–8 weeks, based on a 12-week interim PSMA PET/CT [1307]. The primary endpoint was PSA PFS. Overall, 83 men were assigned to the enzalutamide plus 177Lu-PSMA-RLT group, and 79 were assigned to enzalutamide alone. Median PSA PFS was 13.0 months (95% CI: 11.0–17.0) in the enzalutamide plus RLT group and 7.8 months (95% CI: 4·3–11·0) in the enzalutamide group (HR 0.43, 95% CI: 0.29–0.63, p < 0·0001). The most common AEs were fatigue (75%), nausea (47%), and dry mouth (40%) in the enzalutamide plus RLT and fatigue (70%), nausea (27%], and constipation (23%) in the enzalutamide group. EnzaP suggests that the addition of ¹⁷⁷Lu-PSMA-617 to enzalutamide improved PFS by enhanced anticancer activity in patients with mCRPC [1307]. The actual benefit of the combined use in particular in patients pretreated by one or two ARPIs is still to be proven in larger prospective controlled trials and a firm recommendation would be premature.
6.7.8.3. PARP inhibitors for mCRPC
So far, two PARP inhibitors as monotherapy, olaparib and rucaparib, are licenced by the FDA (EMA only approved olaparib) and several other PARP inhibitors are under investigation or were approved only in combination with an ARPI (see section 6.7.6.5 e.g., talazoparib, niraparib).
A randomised phase III trial (PROfound) compared the PARP inhibitor olaparib to an alternative ARPI in mCRPC with alterations in ≥ 1 of any qualifying gene with a role in HRR and progression on an ARPI. Most patients were heavily pre-treated with 1–2 chemotherapies and up to 2 ARPIs [273,1225]. Radiographic PFS by blinded independent central review in the BRCA1/2 or ATM mutated population (Cohort A) was the first endpoint and significantly favoured olaparib (HR: 0.49, 95% CI: 0.38–0.63). The final results for OS demonstrated a significant improvement among men with BRCA1/2 or ATM mutations (Cohort A) (p = 0.0175; HR: 0.69, 95% CI: 0.50– 0.97). This was not significant in men with any (other) HRR alteration (Cohort B) (HR: 0.96, 95% CI: 0.63–1.49). Of note, patients in the physician’s choice of enzalutamide/abiraterone-arm who progressed, 66% (n = 86/131) crossed over to olaparib.
The most common AEs were anaemia (46.1% vs. 15.4%), nausea (41.4% vs. 19.2%), decreased appetite (30.1% vs. 17.7%) and fatigue (26.2% vs. 20.8%) for olaparib vs. enzalutamide/abiraterone. Among patients receiving olaparib 16.4% discontinued treatment secondary to an AEs, compared to 8.5% of patients receiving enzalutamide/abiraterone. Interestingly, 4.3% of patients receiving olaparib had a pulmonary embolism, compared to 0.8% among those receiving enzalutamide/abiraterone, none of which were fatal. There were no reports of myelodysplastic syndrome or acute myeloid leukaemia. This was the first trial to show a benefit for genetic testing and precision medicine in mCRPC.
The olaparib approval by the FDA is for patients with deleterious or suspected deleterious germline- or somatic HRR gene-mutated mCRPC, who have progressed following prior treatment with enzalutamide or abiraterone. The EMA approved olaparib for patients with BRCA1 and BRCA2 alterations [1308]. The recommended olaparib dose is 600 mg daily (300 mg taken orally twice daily), with or without food.
Rucaparib has been approved by the FDA for patients with deleterious BRCA mutations (germline and/or somatic) who have been treated with ARPI and a taxane-based chemotherapy [1309]. Approval was based on the results of the single-arm TRITON2 trial (NCT02952534). The confirmed ORR per independent radiology review in 62 patients with deleterious BRCA mutations was 43.5% (95% CI: 31–57) [1310]. Rucaparib second line after ARPI was studied in the TRITON 3 trial and is discussed in section 6.7.7.5
The combination of ARPI plus a PARP inhibitor in first-line mCRPC was studied in several RCT including AAP plus Olaparib [1226], AAP plus Niraparib [1230] and Enzalutamide plus Talazoparib [1233]. See Table 6.7.2.
6.7.8.4. Sequencing treatment
6.7.8.4.1. ARPI -> ARPI (chemotherapy-naïve mCRPC patients)
The use of sequential ARPIs in mCRPC showed limited benefit in retrospective series as well as in one prospective trial [1311-1318]. In particular in patients who had a short response to the first ARPI for mCRPC (< twelve months), this sequence should be avoided because of known cross resistance and the availability of chemotherapy and PARP inhibitors (if a relevant mutation is present). In the control arm of the contemporary PSMAfore trial, the ARPI-switch showed an rPFS of 5·59 mo (4.21–5.95) [1284].
In highly selected patients treated for more than 24 weeks with AAP, the sequence with enzalutamide showed some activity with a median rPFS of 8.1 months (95% CI: 6.1–8.3) and an unconfirmed PSA response rate of 27% [1239]. In case the patient is unfit for chemotherapy and a PARP inhibitor, best supportive care should be considered in case no other appropriate treatment option is available (clinical trial or immunotherapy if MSI-high). An ARPI-ARPI sequence should never be the preferred option but might be considered in such patients if the PS still allows for active treatment and the potential side effects seem manageable.
First prospective cross-over data on an ARPI-ARPI sequence [1311] and a SR and meta-analysis suggest that for the endpoints PFS and PSA PFS, but not for OS, abiraterone followed by enzalutamide is the preferred choice [1319].
6.7.8.4.2. ARPI -> PARP inhibitor
This sequence in patients with deleterious or suspected deleterious germline or somatic HRR gene-mutated mCRPC is supported by data from the randomised phase III PROfound trial studying olaparib [1225] and TRITON 3 studying rucaparib [1243]. A subgroup of patients in pROfound was pre-treated with one or two ARPIs and no chemotherapy (35%).
The ARPI-PARP inhibitor sequence versus ARPI-ARPI or ARPI-docetaxel in patients with BRCA 1/ 2 (and ATM) altered tumours was studied in TRITON-3 and showed a significant rPFS benefit in favour of the PARP inhibitor following the first ARPI. These data underscore the importance of early genomic testing in mCRPC patients. (see also chapter 6.7.7.5)
6.7.8.4.3. Docetaxel for mHSPC -> docetaxel rechallenge
There is limited evidence for second- or third-line use of docetaxel after treatment with docetaxel for mHSPC. Docetaxel seems to be less active than ARPI at progression to mCRPC following docetaxel for mHSPC [1320].
6.7.8.4.4. ARPI -> docetaxel or docetaxel -> ARPI followed by PARP inhibitor
Both olaparib and rucaparib are active in biomarker-selected mCRPC patients after ARPI and docetaxel in either sequence [1225,1309].
6.7.8.4.5. ARPI before or after docetaxel
There is level 1 evidence for both sequences (see Table 6.7.3).
6.7.8.4.6. ARPI –> docetaxel -> cabazitaxel or docetaxel –> ARPI -> cabazitaxel
Both third-line treatment Both third-line treatment sequences are supported by level 1 evidence. Of note, there is high-level evidence favouring cabazitaxel vs. a second ARPI after docetaxel and one ARPI in particular in patients progressing ≤ 12 months on a prior ARPI. CARD is the first prospective randomised phase III trial addressing this question (Table 6.7.3) [1237].
6.7.8.5. Platinum chemotherapy
Cisplatin or carboplatin as monotherapy or combinations have shown limited activity in unselected patients in the pre-docetaxel era [1321]. The combination of cabazitaxel and carboplatin was evaluated in pre-treated mCRPC patients in a randomised phase I/II trial. The combination improved the median PFS from 4.5 months (95% CI: 3.5–5.7) to 7.3 months (95% CI: 5.5–8.2; HR: 0.69, 95% CI: 0.50–0.95, p = 0.018) and the combination was well tolerated [1322]. On a histopathological and molecular level, there is preliminary evidence that platinum adds efficacy in patients with aggressive variant PCa molecular signatures including TP53, RB1, and PTEN [1323].
Patients with mCRPC and alterations in DDR genes are more sensitive to platinum chemotherapy than unselected patients [1324], also after progression on PARP inhibitors. Interestingly, in contemporary retrospective series, unselected patients as well as patients without DDR gene alterations also showed a 50% PSA decline when treated with platinum in up to 36% of patients [1297].
In a MA of 23 studies with 901 BRCA-positive mCRPC patients the PSA 50 response rates for PARPi and platinum were 69% (CI: 53–82%), and 74% (CI: 49–90%), respectively. Analyses of OS data showed no difference between PARPi and platinum treatments (HR: 0.86; CI: 0.49-1.52; p = 0.6) [1325]. This analysis supports the use of platinum in patients with BRCA alterations in particular after progression on PARPi or if PARPi are unavailable or suspended due to AEs
In view of the excellent tolerability of e.g., carboplatin monotherapy, platinum could be offered to patients with far advanced mCRPC harbouring DDR gene aberrations after having progressed on standard treatment options. Prospective controlled trials are ongoing.
Table 6.7.1: Randomised phase III controlled trials – nmCRPC
| Study | Intervention | Comparison | Selection criteria | Main outcomes |
ARAMIS | ADT + darolutamide | ADT + placebo | nmCRPC; baseline PSA ≥ 2 ng/mL PSA-DT ≤ 10 mo | 59% reduction of distant progression or death Median MFS: darolutamide 40.4 vs. placebo 18.4 mo; 31% reduction in risk of death HR = 0.69 (95% CI: 0.53–0.88) p = 0.003 |
PROSPER | ADT + enzalutamide | ADT + placebo | nmCRPC; baseline PSA ≥ 2 ng/mL PSA-DT ≤ 10 mo | 71% reduction of distant progression or death Median MFS: enzalutamide 36.6 vs. placebo 14.7 months; 27% reduction in risk of death HR = 0.73 (95% CI: 0.61–0.89) p = 0.001 |
SPARTAN | ADT + apalutamide | ADT + placebo | nmCRPC; baseline PSA ≥2 ng/mL PSA-DT ≤ 10 mo | 72% reduction of distant progression or death Median MFS: apalutamide 40.5 vs. placebo 16.2 months; 22% reduction in risk of death HR = 0.78 (95% CI: 0.64–0.96) p = 0.0161 |
ADT = androgen-deprivation therapy; CI = confidence interval; HR = hazard ratio; MFS = metastasis-free survival; nmCRPC = non-metastatic castrate-resistent prostate cancer; PSA-DT = prostate-specific antigen doubling time.
Table 6.7.2: Randomised phase III controlled trials - first-line treatment of mCRPC
| Study | Intervention | Comparison | Selection criteria | Main outcomes |
| DOCETAXEL | ||||
| SWOG 99-16 2004 [1329] | docetaxel/EMP, every 3 weeks, 60 mg/m2, EMP 3 x 280 mg/day | mitoxantrone, every 3 weeks, 12 mg/m2 prednisone 5 mg BID | OS: 17.52 vs. 15.6 mo. (p = 0.02, HR: 0.80; 95% CI: 0.67–0.97) PFS: 6.3 vs. 3.2 mo. (p < 0.001) | |
| TAX 327 2004, 2008 [1260,1261] | docetaxel, every 3 weeks, 75 mg/m2 prednisone 5 mg BID or docetaxel, weekly, 30 mg/m2 prednisone 5 mg BID | mitoxantrone, every 3 weeks, 12 mg/m2, prednisone 5 mg BID | OS: 19.2 for 3-weekly vs. 17.8 mo. 4-weekly and 16.3 in the control group. (p = 0.004, HR: 0.79, 95% CI: 0.67–0.93) | |
| ABIRATERONE | ||||
| COU-AA-302 2013, 2014, 2015 [1252,1253,1330] | abiraterone + prednisone | placebo + prednisone | - No previous docetaxel - ECOG 0–1 - PSA or radiographic progression - No or mild symptoms. - No visceral metastases | OS: 34.7 vs. 30.3 mo. (HR: 0.81, p = 0.0033) FU: 49.2 mo. rPFS: 16.5 vs. 8.3 mo. (p < 0.0001) |
| ENZALUTAMIDE | ||||
| PREVAIL 2014 [1255] | enzalutamide | placebo | - No previous docetaxel - ECOG 0–1 - PSA or radiographic progression - No or mild symptoms - 10% had visceral mets | OS: 32.4 vs. 30.2 mo. (p < 0.001). FU: 22 mo. (p < 0.001 HR: 0.71, 95% CI: 0.60–0.84) rPFS: 20.0 mo. vs. 5.4 mo. HR: 0.186 (95% CI: 0.15–0.23) p < 0.0001) |
| SIPULEUCEL-T | ||||
| IMPACT 2010 [1266] | sipuleucel-T | placebo | - Some with previous docetaxel - ECOG 0–1 - Asymptomatic or minimally symptomatic | OS: 25.8 vs. 21.7 mo. (p = 0.03 HR: 0.78, 95% CI: 0.61–0.98). FU: 34.1 mo. PFS: 3.7 vs. 3.6 mo. (no difference) |
| 2006 [1331] | sipuleucel-T | - ECOG 0–1 - No visceral met. - No corticosteroids | OS: 25.9 vs. 21.4 mo. (p = 0.1) FU: 36 mo. PFS: 11.7 vs. 10.0 wk. | |
| COMBINATIONS | ||||
| PROpel [1226,1227] | olaparib (300mg BID) + abiraterone (1000 mg/d) + prednisone (5 mg BID) | placebo + abiraterone + prednisone | - ECOG 0-1 - regardless of HRRm (retrospective testing) - prior taxane for mHSPC allowed | ibPFS in ITT population: 24.8 vs. 16.6 mo; HR: 0.66; 95% CI: 0.54–0.81; (p = 0.001) ibPFS in BRCA+: HR 0.24; 95% CI: 0.12- 0.45 OS in ITT population: 42.1 vs. 38.9 mo; HR 0.81; 0.95% CI: 0.81, 0.67-1.0; (p= 0,054)OS in BRCA+: HR 0.30; 95% CI: 0.15- 0.59 |
| niraparib 200 mg/d + abiraterone (1,000 mg/d plus prednisone 5 mg BID) | placebo + abiraterone (1,000 mg/d plus prednisone 5 mg BID) | - ECOG 0-1 - AAP ≤ 4mo allowed for mCRPC - HRR-biomarker positive cohort - prior docetaxel for mHSPC allowed - prior ARPI for mHSPC allowed - prior ARPI for mCRPC allowed | rPFS (central review) in HRR+: 16.5 vs. 13.7 mo HR = 0.73; 95% CI: 0.56-0.96; (p = 0.022) rPFS (central review) in BRCA 1/ 2+: rPFS 19.5 versus 10.9 months; HR= 0.55; 95% CI 0.39-0.78; (nominal p= 0.0007) |
| TALAPRO-2 [1233,1240,1268] | talazoparib (0.5mg/d) + enzalutamide 160mg/d | enzalutamide + placebo | - ECOG 0-1 - All-comers: HHR deficient and HRR non-deficient or unknown - prior AAP or docetaxel allowed for mHSPC | rPFS in ITT: NR (27.5-NR) vs. 21.9 mo; HR 0.63; 95% CI: 0.51-0.78 (p<0.0001); rPFS in BRCA+: HR 0.23; 95% CI: 0.10- 0.53 p=0.0002 |
BID = twice a day; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; EMP = estramustine; FU = follow-up; HR = hazard ratio; mets. = metastases; mo = month; ib (imaging based); (r)PFS = (radiographic) progression-free survival; OS = overall survival; IHC = immunohistochemistry ; HRRm = homologour recombination repair genes mutation; BRCA+ = BRCA gene mutated; ITT = intention to treat; BICR = blinded independent central review.
Table 6.7.3: Randomised controlled phase II/III - second-line/third-line trials in mCRPC
| Randomised controlled phase II/III - second-line/third-line trials in mCRPC | ||||
| Study | Intervention | Comparison | Selection criteria | Main outcomes |
| ABIRATERONE | ||||
COU-AA-301 2012 [1277] | abiraterone + prednisone HR | placebo + prednisone | - Previous docetaxel - ECOG 0–2 - PSA or radiographic progression | OS: 15.8 vs. 11.2 mo. (p < 0.0001, HR: 0.74; 95% CI: 0.64–0.86; p < 0.0001). FU: 20.2 mo. rPFS: no change |
COU-AA-301 2011 [1276] | OS: 14.8 vs. 10.9 mo. (p < 0.001 HR: 0.65; 95% CI: 0.54–0.77). FU: 12.8 mo. rPFS: 5.6 vs. 3.6 mo. | |||
| Radium-223 | ||||
ALSYMPCA 2013 [1279] | radium-223 | placebo | - Previous or no previous docetaxel - ECOG 0–2 - Two or more symptomatic bone metastases - No visceral metastases | OS: 14.9 vs. 11.3 mo. (p = 0.002, HR: 0.61; 95% CI: 0.46–0.81). All secondary endpoints show a benefit over best SOC. |
| CABAZITAXEL | ||||
TROPIC 2013 [1333] | cabazitaxel + prednisone | mitoxantrone + prednisone | - Previous docetaxel - ECOG 0–2 | OS: 318/378 vs. 346/377 events (OR: 2.11; 95% CI: 1.33–3.33). FU: 25.5 months OS ≥ 2 yr. 27% vs. 16% PFS |
TROPIC 2010 [1272] | OS: 15.1 vs. 12.7 mo. (p < 0.0001, HR: 0.70; 95% CI: 0.59–0.83). FU: 12.8 mo. PFS: 2.8 vs. 1.4 mo. (p < 0.0001, HR: 0.74, 95% CI: 0.64–0.86) | |||
CARD 2019 [1237] | cabazitaxel (25 mg/m2 Q3W) + prednisone + G-CSF | ARPI: abiraterone + prednisone OR Enzalutamide | - Previous docetaxel - Progression ≤ 12 mo. on prior alternative ARPI (either before or after docetaxel) | Med OS 13.6 vs. 11.0 mo. (p = 0.008, HR: 0.64, 95% CI: 0.46–0.89). rPFS 8.0 vs. 3.7 mo. (p < 0.001, HR: 0.54, 95% CI: 0.40–0.73). FU: 9.2 mo. |
| ENZALUTAMIDE | ||||
AFFIRM 2012 [1278] | enzalutamide | Placebo | - Previous docetaxel. - ECOG 0–2. | OS: 18.4 vs. 13.6 mo. (p < 0.001, HR: 0.63; 95% CI: 0.53–0.75). FU: 14.4 mo. rPFS: 8.3 vs. 2.9 mo. (HR: 0.40; 95% CI: 0.35–0.47, |
| PARP inhibitor | ||||
PROfound | olaparib | abiraterone + prednisolone or enzalutamide; cross-over allowed at progression | - Previous ARPI, alterations in HRR genes | rPFS: 7.39 vs. 3.55 mo. (p < 0.0001, HR: 0.34; 95% CI: 0.25–0.47), conf. ORR 33.3% vs. 2.3% (OR 20.86, 95% CI: 4.18–379.18). OS: 19.1 mo vs. 14.7 mo (in patients with BRCA1/2, ATM alterations) (p = 0.0175; HR 0.69; 95% CI: 0.5–0.97). |
| TRITON-3 [1243] | rucaparib (600 mg BID) | docetaxel or abiraterone acetate or enzalutamide | - EOCG 0-1 - Previous one ARPI - BRCA 1/2 or ATM alteration | rPFS: ITT 10.2 mo vs. 6.4 mo, (HR 0.61; 95% CI, 0.47 to 0.80; p < 0.001 for both comparisons) |
| Radioligand therapy | ||||
VISION 2021 [1302] | 177Lu-PSMA-617 SOC | SOC alone | - Previous at least 1 ARPI and one or two taxane regimens; - Mandatory: PSMA-positive gallium-68 (68Ga)–labelled PSMA-PET scan | Imaging-based PFS: 8.7 vs. 3.4 mo. (p < 0.001; HR 0.40; 99.2% CI: 0.29–0.57) OS: 15.3 vs. 11.3 mo. (p < 0.001; HR 0.62; 95% CI: 0.5–0.74) |
TheraP | 177Lu-PSMA-617 (8.5 GBq i.v.q 6-weekly, decreasing 0.5 GBq/cycle; up to 6 cycles) | 177Lu-PSMA-617 1:1 randomisation cabazitaxel (20 mg/m2 i.v.q 3-weekly, up to 10 cycles) | - Post docetaxel, - Suitable for cabaziaxel | First endpoint PSA reduction of > 50%: 66 vs. 37 PSA responses; 66% vs. 37% by ITT; difference 29% (95% CI: 16–42; p < 0.0001; and 66% vs. 44% by treatment received; difference 23% [9–37]; p = 0.0016). Secondary endpoint OS: 19.1 vs. 19.6 mo (177Lu-PSMA vs. cabazitaxel). HR: 0.97, 95% CI: 0.7-1.4 (p = 0.99) |
| PSMAfore 2023 [1284] | 177Lu-PSMA-617 at a dosage of 7·4 GBq (200 mCi)1398042195±139804219510%; 6 cycles | 177Lu-PSMA-617 1:1 randomisation to ARPI- change (abiraterone or enzalutamide) | - One previous ARPI for mCRPC - No previous taxane in CRPC or HSPCb | First endpoint: rPFS 3rd data-cut-off : 11·60 mo (95% CI 9·30–14·19) vs 5·59 mo (4·21–5·95) (HR 0·49 [95% CI 0·39–0·61]) |
*Only studies reporting survival outcomes as primary endpoints have been included.ARPI = androgen receptor pathway inhibitor; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; FU = follow-up; GBq = gigabecquerel; HR = hazard ratio; Lu = lutetium; mo = months OS = overall survival; OR = odds ratio; ORR = objective response rate; PSA = prostate-specific antigen; PSMA = prostatespecific membrane antigen; (r)PFS = (radiographic) progression-free survival; SOC = standard of care; yr = year; HRR= homologous recombination repair.
6.7.9. Monitoring of treatment
Baseline examinations should include a medical history, clinical examination as well as baseline blood tests (PSA, total testosterone level, full blood count, renal function, baseline liver function tests, alkaline phosphatase), bone scan and CT of chest, abdomen and pelvis [1334,1335]. The use of choline or PSMA PET/CT scans for progressing CRPC is unclear and most likely not as beneficial as for patients with BCR or hormone-naive disease. Flares, PSMA upregulation and discordant results compared with PSA response or progression on ARPI have been described [1336]. Prostate-specific antigen alone is not reliable enough [1337] for monitoring disease activity in advanced CRPC since visceral metastases may develop in men without rising PSA [1338]. Instead, the PCWG2 recommends a combination of bone scintigraphy and CT scans, PSA measurements and clinical benefit in assessing men with CRPC [1218]. A majority of experts at the 2015 Advanced Prostate Cancer Consensus Conference (APCCC) suggested regular review and repeating blood profile every two to three months with bone scintigraphy and CT scans at least every six months, even in the absence of a clinical indication [1334]. This reflects that the agents with a proven OS benefit all have potential toxicity and considerable cost and patients with no objective benefit should have their treatment modified. The APCCC participants stressed that such treatments should not be stopped for PSA progression alone. Instead, at least two of the three criteria (PSA progression, radiographic progression and clinical deterioration) should be fulfilled to stop treatment. For trial purposes, the updated PCWG3 put more weight on the importance of documenting progression in existing lesions and introduced the concept of no longer ‘clinically benefiting‘ to underscore the distinction between first evidence of progression and the clinical need to terminate or change treatment [1218]. These recommendations also seem valid for clinical practice outside trials.
6.7.10. When to change treatment
The timing of treatment change for men with metastatic prostate cancer remains a matter of debate in although it is clearly advisable to start or change treatment immediately in men with symptomatic progressing metastatic disease. Preferably, any treatment change should precede development of de novo symptoms or worsening of existing symptoms. Although, the number of effective treatments is increasing, head-to-head comparisons are still rare, as are prospective data assessing the sequencing of available agents. Therefore it is not clear how to select the most appropriate ‘second-line‘ treatment, in particular in patients without HRR alterations or other biomarkers. A positive example, however, is the CARD trial which clearly established cabazitaxel as the better third-line treatment in docetaxel pre-treated patients after one ARPI compared to the use of a second ARPI [1237].
The ECOG PS has been used to stratify patients. Generally men with a PS of 0–1 are likely to tolerate treatments and those with a PS of > 2 are less likely to benefit. However, it is important that treatment decisions are individualised, in particular when symptoms related to disease progression are impacting on PS. In such cases, a trial of active life-prolonging agents to establish if a given treatment will improve the PS may be appropriate. Sequencing of treatment is discussed in the summary papers published following the 2019 and 2022 APCCC Conferences [1339,1340].
6.7.11. Symptomatic management in metastatic castration-resistant prostate cancer
Castration-resistant PCa is usually a debilitating disease often affecting the elderly male. A multidisciplinary approach is required with input from urologists, medical oncologists, radiation oncologists, nurses, psychologists and social workers [1339,1341]. Critical issues of palliation must be addressed when considering additional systemic treatment, including management of pain, constipation, anorexia, nausea, fatigue and depression.
6.7.11.1. Common complications due to bone metastases
Most patients with CRPC have painful bone metastases. External beam RT is highly effective, even as a single fraction [1342,1343]. A single infusion of a third-generation bisphosphonate could be considered when RT is not available [1344]. Common complications due to bone metastases include vertebral collapse or deformity, pathological fractures and spinal cord compression. Cementation can be an effective treatment for painful spinal fracture whatever its origin, clearly improving both pain and QoL [1345]. It is important to offer standard palliative surgery, which can be effective for managing osteoblastic metastases [1346,1347]. Impending spinal cord compression is an emergency. It must be recognised early and patients should be educated to recognise the warning signs. Once suspected, high-dose corticosteroids must be given and MRI performed as soon as possible. A systematic neurosurgery or orthopaedic surgeon consultation should be planned to discuss a possible decompression, followed by EBRT [1348]. Otherwise, EBRT with, or without, systemic therapy, is the treatment of choice.
6.7.11.2. Preventing skeletal-related events
6.7.11.2.1. Bisphosphonates
Zoledronic acid has been evaluated in mCRPC to reduce skeletal-related events (SRE). This study was conducted when no active anti-cancer treatments, but for docetaxel, were available. Six hundred and forty three patients who had CRPC with bone metastases were randomised to receive zoledronic acid, 4 or 8 mg every three weeks for fifteen consecutive months, or placebo [1349]. The 8 mg dose was poorly tolerated and reduced to
4 mg but did not show a significant benefit. However, at fifteen and 24 months of follow-up, patients treated with 4 mg zoledronic acid had fewer SREs compared to the placebo group (44 vs. 33%, p = 0.021) and in particular fewer pathological fractures (13.1 vs. 22.1%, p = 0.015). Furthermore, the time to first SRE was longer in the zoledronic acid group. No survival benefit has been seen in any prospective trial with bisphosphonates.
6.7.11.2.2. RANK ligand inhibitors
Denosumab is a fully human monoclonal antibody directed against RANKL (receptor activator of nuclear factor κ-B ligand), a key mediator of osteoclast formation, function, and survival. In M0 CRPC, denosumab has been associated with increased bone-MFS compared to placebo (median benefit: 4.2 months, HR: 0.85, p = 0.028) [1342]. This benefit did not translate into a survival difference (43.9 compared to 44.8 months, respectively) and neither the FDA or the EMA have approved denosumab for this indication [1350].
The efficacy and safety of denosumab (n = 950) compared with zoledronic acid (n = 951) in patients with mCRPC was assessed in a phase III trial. Denosumab was superior to zoledronic acid in delaying or preventing SREs as shown by time to first on-study SRE (pathological fracture, radiation or surgery to bone, or spinal cord compression) of 20.7 vs. 17.1 months, respectively (HR: 0.82, p = 0.008). Both urinary N-telopeptide and bone-specific alkaline phosphatase were significantly suppressed in the denosumab arm compared with the zoledronic acid arm (p < 0.0001 for both). However, these findings were not associated with any survival benefit and in a post-hoc re-evaluation of endpoints, denosumab showed identical results when comparing SREs and symptomatic skeletal events [1351].
The potential toxicity (e.g., osteonecrosis of the jaw, hypocalcaemia) of these drugs must always be kept in mind (5–8.2% in M0 CRPC and mCRPC, respectively) [1352,1353]. Patients should have a dental examination before starting therapy as the risk of jaw necrosis is increased by several risk factors including a history of trauma, dental surgery or dental infection [1354]. Also, the risk for osteonecrosis of the jaw increased numerically with the duration of use in a pivotal trial [1355] (one year vs. two years with denosumab), but this was not statistically significant when compared to zoledronic acid [1350]. According to the EMA, hypocalcaemia is a concern in patients treated with denosumab and zoledronic acid. Hypocalcaemia must be avoided by adequate intake of calcium and vitamin D before initiating therapy [1356]. Hypocalcaemia should be identified and prevented during treatment with bone protective agents (risk of severe hypocalcaemia is 8% and 5% for denosumab and zoledronic acid, respectively) [1353]. Serum calcium should be measured in patients starting therapy and monitored during treatment, especially during the first weeks and in patients with risk factors for hypocalcaemia or on other medication affecting serum calcium. Daily calcium (> 500 mg) and vitamin D (> 400 IU equivalent) are recommended in all patients, unless in case of hypercalcaemia [1353,1357,1358].
6.7.12. Summary of evidence and recommendations for life-prolonging treatments of castrate-resistant disease
| Summary of evidence | LE |
| Treatment for mCRPC will be influenced by which treatments patients have already been exposed to. | 4 |
| Recommendations | Strength rating |
| Ensure that testosterone levels are confirmed to be < 50 ng/dL before diagnosing castrate-resistant PCa (CRPC). | Strong |
| Counsel, manage and treat patients with metastatic CRPC (mCRPC) in a multidisciplinary team. | Strong |
| Treat patients with mCRPC with life-prolonging agents. | Strong |
| Offer mCRPC patients somatic and/or germline molecular testing as well as testing for mismatch repair deficiencies or microsatellite instability. | Strong |
6.7.13. Recommendations for systemic treatments of castrate-resistant disease
Summary statement for mCRPC first line combination therapy: The combination of ARPI plus PARP inhibitors showed a significant rPFS benefit in RCTs for unselected patients. However, this benefit is mainly driven by HRR- and even more pronounced by BRCA 1/2- altered patients. So far, no clear OS benefit was seen, and the side effects of PARP inhibitors add substantial toxicity to ARPI monotherapy. Therefore, no recommendation is given for patients without HRR or BRCA 1/2 -mutations and the data will be re-evaluated after longer follow-up.
| Recommendations | Strength rating |
| Base the choice of treatment on the performance status (PS), symptoms, co-morbidities, location and extent of disease, genomic profile, patient preference, and on previous treatment for hormone-sensitive metastatic PCa (mHSPC) (alphabetical order: abiraterone, cabazitaxel, docetaxel, enzalutamide, 177lutetium-PSMA-617-radioligand therapy, radium-223, sipuleucel-T, and for patients with DNA homologous recombination repair (HRR) alterations olaparib, olaparib/abiraterone, niraparib/abiraterone, rucaparib, talazoparib/enzalutamide). | Strong |
| Avoid sequencing of androgen receptor targeted agents. | Strong |
| Offer chemotherapy to patients previously treated with abiraterone or enzalutamide. | Strong |
| Offer patients with metastatic castrate-resistant PCa (mCRPC) who are candidates for cytotoxic therapy and are chemotherapy naïve docetaxel with 75 mg/m2 every three weeks. | Strong |
| Offer patients previously untreated for mCRPC and harbouring an HRR or BRCA mutation abiraterone in combination with olaparib if the patient is fit for both agents and did not previously receive an ARPI. | Strong |
| Offer patients previously untreated for mCRPC and harbouring a BRCA mutation abiraterone in combination with niraparib if the patient is fit for both agents and did not previously receive an ARPI. | Strong |
| Offer patients previously untreated for mCRPC and harbouring an HRR-mutation enzalutamide in combination with talazoparib if the patient is fit for both agents and did not previously receive an ARPI. | Strong |
| Offer poly(ADP-ribose) polymerase (PARP) inhibitors to pre-treated mCRPC patients with relevant DNA repair gene mutations. | Strong |
| Offer patients with mCRPC and progression following docetaxel chemotherapy further life-prolonging treatment options, which include abiraterone, cabazitaxel, enzalutamide, radium-223 and olaparib in case of DNA HRR alterations. | Strong |
| Base further treatment decisions of mCRPC on PS, previous treatments, symptoms, co-morbidities, genomic profile, extent of disease and patient preference. | Strong |
| Offer abiraterone or enzalutamide to patients previously treated with one or two lines of chemotherapy. | Strong |
| Offer cabazitaxel to patients previously treated with docetaxel. | Strong |
| Offer cabazitaxel to patients previously treated with docetaxel who have progressed within twelve months of treatment with abiraterone or enzalutamide for mCRPC. | Strong |
| Offer 177Lu-PSMA-617 to pre-treated mCRPC patients with one or more metastatic lesions, highly expressing PSMA (exceeding the uptake in the liver) on the diagnostic radiolabelled PSMA PET/CT scan. | Strong |
6.7.14. Guideline for non-metastatic castrate-resistant disease
| Recommendation | Strength rating |
| Offer apalutamide, darolutamide or enzalutamide to patients with M0 CRPC and a high risk of developing metastasis (PSA-DT < 10 months) to prolong time to metastases and overall survival. | Strong |
Figure 6.4: Treatment non-metastasized (M0) – asymptomatic disease

* Rule of thumb: Life expectancy ten years.
** Recommendation based on clinical staging using digital rectal examination, not imaging.
*** Recommendation based on staging using combination of bone scan and CT.
**** See text, dependent on GG and (biopsy) volume.
1 EBRT: IMRT/VMAT + IGRT of the prostate.
Light green = weak recommendation. ADT = androgen deprivation therapy; EBRT =external beam radiotherapy; ECE = extracapsular extension; ePLND = extended pelvic lymph node dissection; GG = grade group; HDR = high-dose rate; IDC = intraducal carcinoma; IGRT = image-guided radiotherapy; IMRT = intensity-modulated radiotherapy; LDR = low-dose rate; VMAT = volumetric modulated arc therapy.
Figure 6.5: Treatment of metastasized (M1*) – disease, M+HSPC
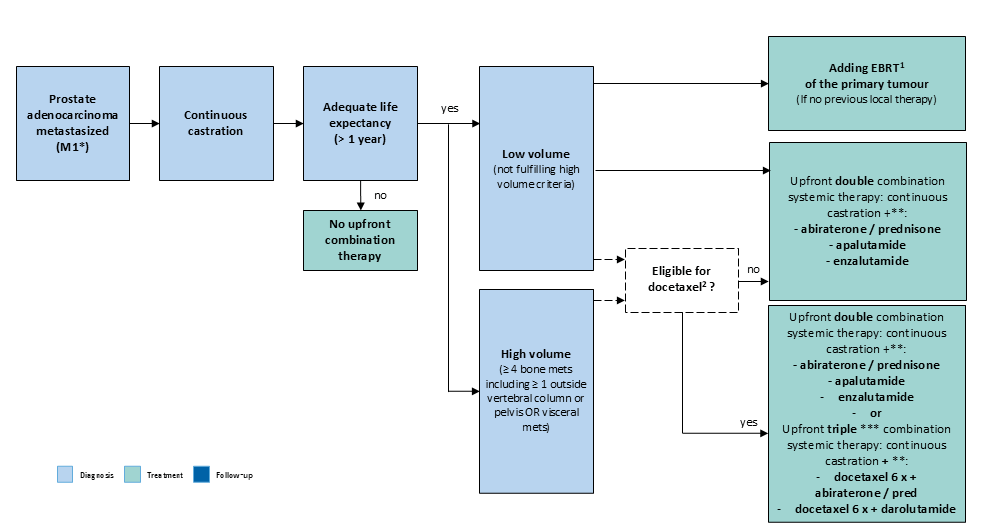
* Based on staging using combination of bone scan and CT.
** Alphabetical order.
***not for low volume, metachronous disease.
1 EBRT: IMRT/VMAT + IGRT of the prostate (equivalent of up to 72 Gy in 2 Gy fractions).
2 Triple therapy was better than ADT plus docetaxel but randomised data comparing it to ADT plus ARTA is missing.
EBRT = external beam radiotherapy; IGRT = image-guided radiotherapy; IMRT = intensity-modulated radiotherapy.
#Note: Please be aware that the various options in the following flowcharts present a generalised approach only, and cannot take the management of individual patients into account, nor the availability of resources.