3. THE GUIDELINE
3.1. Classification
Urinary tract infections encompass a wide spectrum of clinical and pathologic conditions affecting various parts of the urinary tract. Each condition has its own unique epidemiology, natural history, and diagnostic considerations. A precise distinction is crucial as it significantly impacts treatment and prognosis. Therefore, standardised terminology is essential for effective communication on this subject. Different classification systems of UTI exist. The most widely used systems are from the Centres for Disease Control and Prevention (CDC) [5], the Infectious Diseases Society of America (IDSA) [6], the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) [7] and the U.S. Food and Drug Administration (FDA) [8,9]. Current guidelines often differentiate between uncomplicated and complicated UTIs, with some modifications. According to these definitions, uncomplicated UTIs occur in healthy, non-pregnant women, while all other UTIs fall into the category of complicated UTIs. This classification is straightforward, yet it carries inherent risks, as it can significantly affect initial patient management and treatment selection.
The Guidelines Panel has proposed a new classification scheme for UTIs aimed at enhancing consistency in clinical practice and providing a comprehensive framework for understanding various clinical presentations. The proposed classification does not use the terms ’uncomplicated’ and ’complicated’ anymore; instead, it emphasises the difference between localised and systemic UTIs identified by clinical signs and symptoms (Figure 1 and Table 1).
The definitions are as follows (Figure 1):
- Localised UTI (i.e., cystitis): A cystitis without any signs and symptoms of systemic infection in either sex.
- Systemic UTI: An infection with signs and symptoms of systemic infection with or without localised symptoms originating from any site in the urinary tract in either sex.
According to the new definition, UTIs can manifest as either localised (i.e., cystitis) or systemic (e.g., pyelonephritis, prostatitis, etc) infection. Both conditions may be accompanied by risk factors that increase the likelihood of a challenging clinical course and jeopardise treatment success. Clinicians must be aware of these risk factors and should address them accordingly.
This definition allows medical practitioners to distinguish between a localised UTI that can be treated in general on an outpatient basis and a more complex systemic UTI that may necessitate blood sampling, imaging (e.g., ultrasound, cross-sectional imaging), intravenous antimicrobial treatment and hospitalisation.
Figure 1: Classification of UTI 
Table 1: Localised and systematic signs and symptoms of UTI
| Localised UTI1 | Systemic UTI1,2 |
| Dysuria (pain, burning, stinging) | Fever or hypothermia |
| Urgency | Rigors, shaking chills |
| Frequency | Delirium |
| Incontinence | Hypotension |
| Urethral purulence | Tachycardia |
| Pressure or cramping in the lower abdomen | Costovertebral angle pain/tenderness |
1. Recent onset of these localised and/or systemic signs and symptoms.
2. These signs and symptoms are possibly caused by a systemic UTI, but there may be also be alternative explanations.
3.1.1. Risk factors
When managing UTIs, it is essential to consider risk factors that may predispose patients to a severe clinical course or treatment failure. Factors such as urinary tract abnormalities, the presence of urinary catheters, previous antibiotic treatment, and underlying health conditions (e.g., diabetes, renal impairment, and neurological disorders) can significantly influence the outcome of UTIs. Importantly, in the proposed classification male sex not considered as a risk factor, as it lacks support from contemporary literature. Table 2 provides a clear overview of risk factors for clinicians). By identifying and addressing these risk factors early in the treatment process, clinicians can optimise patient care and improve treatment outcomes.
Table 2: UTI Risk Factors*
| UTI Risk Factor | ||
| Infants | Immunocompromised state | Male sex • Prostatic involvement |
| Geriatric or frail patients | Post void residual volume | |
| Anatomic or functional abnormalities of the urinary tract | Neurourological patients | Female sex • Pregnancy • Pelvic organ prolapse |
| Antibiotic use in the past | ||
| Indwelling urinary catheters | Resistant organisms | |
| Stones | Obstruction | Recent instrumentation |
* Both localised and systemic UTIs may be accompanied by risk factors that increase the likelihood of a challenging clinical course and jeopardise treatment success. Clinicians must be aware of these risk factors to adjust treatment if necessary.
3.2. Antimicrobial Stewardship
Although the benefits to patients of antibiotic use are clear, overuse and misuse have contributed to the growing problem of resistance amongst uropathogenic bacteria, which is a serious threat to public health [10,11]. In acute care hospitals, 20-50% of prescribed antibiotics are either unnecessary or inappropriate [12]. In response, a worldwide initiative seeks to incorporate antimicrobial stewardship programmes in healthcare [13]. Antimicrobial Stewardship aims to optimise clinical outcomes and ensure cost-effective therapy whilst minimising unintended consequences of antimicrobial use such as healthcare associated infections including Clostridioides difficile, toxicity, selection of virulent organisms and emergence of resistant bacterial strains [14].
Stewardship programmes have two main sets of actions. The first set mandates use of recommended care at the patient level conforming to guidelines. The second set describes strategies to achieve adherence to the mandated guidance. These include persuasive actions such as education and feedback together with restricting availability linked to local formularies. A Cochrane review of effectiveness of interventions to improve antibiotic prescribing practices for hospital inpatients, updated in 2017, found high-certainty evidence that such interventions are effective in increasing adherence with antibiotic policy leading to reduced antibiotic treatment duration and that it may also reduce hospital stay. The review found no evidence that reduced antibiotic usage increased mortality [15].
The important components of antimicrobial stewardship programmes are [16]:
- regular training of staff in best use of antimicrobial agents;
- adherence to local, national or international guidelines;
- regular ward visits and consultation with infectious diseases physicians and clinical microbiologists;
- audit of adherence and treatment outcomes;
- regular monitoring and feedback to prescribers of their performance and local pathogen resistance profiles.
A 2016 systematic review of evidence for effectiveness of various antimicrobial stewardship interventions in healthcare institutions identified 145 studies of nine stewardship objectives. Guideline-driven empirical therapy using a restricted choice of antibiotics and including de-escalation, intravenous to oral switch, therapeutic drug monitoring, and bedside consultation resulted in a 35% (95% CI: 20-46%) relative risk reduction (RRR) in mortality. Use of de-escalation (tailoring to a more narrow spectrum agent), showed a RRR of 56% (95% CI:
34-70%) for mortality [17].
To facilitate local initiatives and audit, a set of valid, reliable, and applicable indicators of the quality of antibiotic use in the treatment of hospitalised patients with complicated UTI was developed [18]. Its use in the Netherlands appeared to result in shortened hospital stay [19]. A literature search of Pubmed from April 2014 [17], to February 2017 identified no further randomised controlled trials (RCTs) relating to stewardship programmes for UTIs. Studies to provide high-quality evidence of effectiveness of stewardship programmes in urology patients are urgently needed.
3.3. Asymptomatic bacteriuria in adults
3.3.1. Background
Urinary growth of bacteria in an asymptomatic individual (asymptomatic bacteriuria [ABU]) is common, and corresponds to a commensal colonisation [20]. Clinical studies have shown that ABU may protect against superinfecting UTI, thus treatment of ABU should be performed only in cases of proven benefit for the patient to avoid the risk of selecting antimicrobial resistance and eradicating a potentially protective ABU strain [21,22]. The aim of this section is to support the clinician in deciding when ABU should or should not be treated.
3.3.2. Epidemiology, aetiology and pathophysiology
Asymptomatic bacteriuria occurs in an estimated 1-5% of healthy pre-menopausal females. Increasing to 4-19% in otherwise healthy elderly females and men, 0.7-27% in patients with diabetes, 2-10% in pregnant women, 15-50% in institutionalised elderly populations, and in 23-89% in patients with spinal cord injuries [23]. Asymptomatic bacteriuria in younger men is uncommon, but when detected, chronic bacterial prostatitis must be considered. The spectrum of bacteria in ABU is similar to species found in localised or systemic UTIs, depending on the presence of risk factors (see sections 3.4 and 3.7).
3.3.3. Diagnostic evaluation
Asymptomatic bacteriuria is defined by a mid-stream sample of urine showing bacterial growth ≥ 105 cfu/mL in two consecutive samples in women [24] and in one single sample in men [25], in an individual without urinary tract symptoms. Cystoscopy and/or imaging of the upper urinary tract is not mandatory if the medical history is otherwise without remark. If persistent growth of urease producing bacteria, i.e. Proteus mirabilis is detected, stone formation in the urinary tract must be excluded [26-28]. In men, a digital rectal examination (DRE) has to be performed to investigate the possibility of prostate diseases (see section 3.11).
3.3.4. Evidence summary
A systematic search of the literature from November 2016 to January 2000 identified 3,582 titles of which 224 were selected for full text review and 50 were included [29]. For the subgroups of pregnancy, prior to urologic surgeries, post-menopausal women and institutionalised elderly patients, only data from RCTs were included, on which a meta-analysis was performed [29]. For the other subgroups, non-RCTs were also included in the narrative analysis [29]. An update systematic literature search from 1st December 2016 to 1st June 2023 identified 1,503 titles of which 36 were selected for full text review and 18 were included. The following patient populations were not covered by the systematic review: immuno-compromised patients; and patients with indwelling catheters. For these groups, the guideline was updated using a structured PubMed search. The evidence question addressed was: “What is the most effective management for people with asymptomatic bacteriuria?”
3.3.5. Disease management
3.3.5.1. Patients without identified risk factors
Asymptomatic bacteriuria does not cause renal disease or damage [30]. Only one prospective, non-randomised study investigated the effect of treatment of ABU in adult, non-diabetic, non-pregnant women [31], and found no difference in the rate of symptomatic UTIs. Furthermore, as the treatment of ABU has been proven to be unnecessary in most high-risk patient subgroups, there is panel consensus that the results of these subgroups can also be applied to patients without identified risk factors. Therefore, screening and treatment of ABU is not recommended in patients without risk factors.
3.3.5.2. Patients with ABU and recurrent UTI, otherwise healthy
One RCT investigated the effect of ABU treatment in female patients with recurrent symptomatic UTI without identified risk factors [22] and demonstrated that treatment of ABU increases the risk for a subsequent symptomatic UTI episode, compared to non-treated patients (RR 0.28, 95% CI 0.21 to 0.38; n=673). This protective effect of spontaneously developed ABU can be used as part of prevention in female patients with recurrent symptomatic UTI; therefore, treatment of ABU is not recommended.
3.3.5.3. Pregnant women
3.3.5.3.1. Is treatment of ABU beneficial in pregnant women?
Twelve RCTs comparing antibiotic treatments of ABU with placebo controls or no treatment [32-43], with different antibiotic doses and regimens were identified, ten published before 1988 and one in 2015. Eleven RCTs (n=2,002) reported on the rate of symptomatic UTIs [32,34-42,44]. Antibiotic treatment significantly reduced the number of symptomatic UTIs compared to placebo or no treatment (average RR 0.22, 95% CI: 0.12 to 0.40).
Six RCTs reported on the resolution of bacteriuria [32-34,36,39,41]. Antibiotic treatment was effective in the resolution of bacteriuria compared to placebo (average RR 2.99, 95% CI: 1.65 to 5.39; n=716). Eight RCTs reported on the rate of low birthweights [32,34-37,40,43,44]. Antibiotic treatment was associated with lower rates of low birthweight compared to placebo or no treatment (average RR 0.58, 95% CI: 0.36 to 0.94; n=1689). Four RCTs reported on the rate of preterm deliveries [40,41,43,44]. Antibiotic treatment was associated with lower rates of preterm delivery compared to placebo or no treatment (average RR 0.34, 95% CI: 0.18 to 0.66; n=854). Three additional systematic reviews and meta-analyses have reported that treatment of ABU in pregnancy may be associated with a decreased rate of pyelonephritis, low birthweight or preterm delivery [45-47]. However, they also emphasised the low to very low quality of the evidence of the identified studies.
Based on the beneficial maternal and foetal effects of antibiotic treatment, pregnant women should be screened and treated for ABU. However, the panel would like to emphasise that most available studies have low methodological quality and are from the 60s to 80s. Diagnostic and treatment protocols and accessibility to medical services have dramatically changed since then; therefore, the quality of evidence for this recommendation is low. In a newer study of higher methodological quality the beneficial effects of antibiotic treatment are not as evident [44]. Therefore, it is advisable to consult national recommendations for pregnant women.
3.3.5.3.2. Which treatment duration should be applied to treat ABU in pregnancy?
Sixteen RCTs comparing the efficacy of different antibiotic treatments in pregnant women with ABU were identified [48-63]. There was significant heterogeneity amongst the studies. Studies compared different antibiotic regimens or the same antibiotic regimens with different durations. The duration of treatment ranged from single dose to continuous treatment (until delivery). For practical purposes the grouping strategy used by the previously published Cochrane Review was adopted with some modifications [64]. The following treatment groups were used for comparison:
- single dose (single day);
- short course (2-7 days);
- long course (8-14 days);
- continuous (until delivery).
Nine studies compared single dose to short course treatment [49,53,54,58-63], one study compared single dose to long course treatment [57] and one study compared long course to continuous treatment [50]. As long term and continuous antibiotic treatment is not used in current practice, only studies comparing single dose to standard short course treatment are presented.
3.3.5.3.2.1. Single dose vs. short course treatment
Three RCTs reported on the rate of symptomatic UTIs [53,62,63], with no significant difference between the two durations (average RR 1.07, 95% CI: 0.47 to 2.47; n=891). Nine RCTs reported on the rate of ABU resolution [49,53,54,58-63], with no significant difference between the two durations (average RR 0.97, 95% CI: 0.89 to 1.07; n=1,268). Six RCTs reported on the rate of side effects [49,53,58,59,61,62]. Single dose treatment was associated with significantly less side effects compared to short course treatment (average RR 0.40, 95% CI: 0.22 to 0.72; n=458). Three RCTs reported on the rate of preterm deliveries [53,55,63], with no significant difference between the two durations (average RR 1.16, 95% CI: 0.75 to 1.78; n=814). One RCT reported on the rate of low birthweights [63]. There were significantly more babies with low birthweight in the single dose duration compared to short course treatment (average RR 1.65, 95% CI: 1.06 to 2.57; n=714).
According to the data analysis, single dose treatment was associated with a significantly lower rate of side effects but a significantly higher rate of low birthweight. A meta-analysis on the use of single dose fosfomycin trometamol in women with lower uncomplicated UTIs or ABU reported on a sub-group analysis of pregnant women with ABU [65]. The study identified five RCTs involving 577 patients. The resolution rate of ABU in pregnant women treated with single dose fosfomycin trometamol was not significantly different from those who received other antibiotics (OR 1.32, 95% CI: 0.78–2.22, p=0.30). Therefore, standard short course treatment or single dose fosfomycin trometamol should be applied to treat ABU in pregnancy; however, it should be emphasised that the overall quality of the scientific evidence backing this recommendation is low.
3.3.5.4. Patients with identified risk-factors
3.3.5.4.1. Diabetes mellitus
Diabetes mellitus, even when well regulated, is reported to correlate to a higher frequency of ABU [66]. One RCT demonstrated that eradicating ABU did not reduce the risk of symptomatic UTI and infectious complications in patients with diabetes mellitus. The time to first symptomatic episode was also similar in both groups. Furthermore, untreated ABU did not correlate to diabetic nephropathy [67]. Screening and treatment of ABU in well-controlled diabetes mellitus is therefore not recommended. However, poorly regulated diabetes is a risk factor for symptomatic UTI and infectious complications.
3.3.5.4.2. ABU in post-menopausal women
Elderly women have an increased incidence of ABU [68]. Four RCTs compared antibiotic treatment of ABU with placebo controls or no treatment, in a post-menopausal female population, with different antibiotic doses and regimens [69-72]. Women in these studies were mostly nursing home residents, which may bias the results of this analysis. Three RCTs reported on the rate of symptomatic UTIs (average RR 0.71, 95% CI: 0.49 to 1.05; 208 women) and the resolution of bacteriuria (average RR 1.28, 95% CI: 0.50 to 3.24; 203 women) [53,62,63], with no significant benefit of antibiotic treatment. Therefore, ABU in post-menopausal women does not require treatment, and should be managed as for pre-menopausal women.
3.3.5.4.3. Elderly institutionalised patients
The rate of ABU is 15-50% in elderly institutionalised patients [73]. Differential diagnosis of ABU from symptomatic UTI is difficult in the multi-diseased and mentally deteriorated patients, and is probably a cause of unnecessary antibiotic treatment [74,75]. Seven RCTs compared antibiotic treatment of ABU with placebo controls or no treatment in elderly patients, with different antibiotic doses and regimens [69-72,76-78].
Three RCTs reported on the rate of symptomatic UTIs [69,71,76]. Antibiotic treatment was not significantly beneficial in reducing the rate of symptomatic UTIs compared to placebo or no treatment (average RR 0.68, 95% CI: 0.46 to 1.00; n=210). Six RCTs reported on the resolution of bacteriuria [69,71,72,76-78]. There was no benefit of antibiotic treatment compared to placebo in the resolution of ABU (average RR 1.33, 95% CI: 0.63 to 2.79; n=328). One RCT compared the rates of incontinence in this patient group before and after the eradication of ABU and found no effect of antibiotic treatment [79]. A subsequent systematic review and meta-analysis of nine RCTs found that antibiotic treatment of ABU in this group was associated with significantly more adverse effects with no clinical benefit [80]. Therefore, screening and treatment of ABU is not recommended in this patient group.
3.3.5.4.4. Patients with renal transplants
Two RCTs and two retrospective studies compared the effect of antibiotic treatment to no treatment in renal transplant patients [81-84]. Meta-analysis of the two RCTs did not find antibiotic treatment beneficial in terms of reducing symptomatic UTIs between twelve and 22 months after renal transplantation (RR 0.86, 95% CI: 0.51 to 1.45; n=200). The two retrospective studies reached the same conclusion. Furthermore, there were no significant differences in the rate of ABU clearance, graft loss or change in renal function during long-term follow-up up to 24 months [81-84].
A further two RCTs [85,86], one observational study [87] and two systematic reviews and meta-analyses [88,89] were identified. The first RCT reported that during the first two months following renal transplantation the incidence of and risk for UTIs (25% vs. 10%, HR 2.8, 95% CI: 0.8-9.1, p =0.07) and pyelonephritis (15% vs. 2.5%, HR 6.5, 95% CI: 0.8-54.7, p=0.08) was higher in patients receiving antibiotic treatment for ABU vs. no treatment [85]. In the second RCT no difference in acute graft pyelonephritis was found between the treatment and no treatment group (12.2% vs 8.7%, RR 1.40, 95% CI: 0.40-4.87) in the first year after renal transplantation; however, rates of antimicrobial resistance were higher in the treatment group [86]. The first of the two additional meta-analyses reported the same results as the original study [88]. The second meta-analysis of n = 1,353 patients reported ABU incidence rates of 22% in the first month and 32% during the first year after renal transplantation [89]. The analysis did not find a correlation between ABU and acute graft pyelonephritis (OR 1.8, 95% CI: 0.78-1.79), a benefit of ABU antibiotic treatment on the risk of UTI (OR 1.08, 95% CI: 0.63-1.84) or a change of renal function (mean difference in serum creatinine concentration - 0.03 mg/dL [95% CI: 0.15-0.10]) [89]. Therefore, treatment of ABU is not recommended in renal transplant recipients.
3.3.5.4.5. Patients with dysfunctional and/or reconstructed lower urinary tracts
Patients with lower urinary tract dysfunction (LUTD) (e.g., neurogenic lower urinary tract dysfunction (NLUTD) secondary to multiple sclerosis, spinal cord injury patients, patients with incomplete bladder emptying, patients using clean intermittent catheterisation [CIC], or patients with reconstructed lower urinary tract including ileal conduits, orthotopic bladder replacement or continent reservoirs frequently become colonised [90,91]. A systematic review reported ABU prevalence rates ranging from 25-86% for intestinal conduits in four studies and 9.1-85% for orthotopic neobladders in nine studies [92]. Studies have shown no long-term benefit in ABU treatment in these patient groups [84,85,92]. Furthermore, in LUTD patients who do not spontaneously develop ABU, deliberate colonisation with an ABU strain (Escherichia coli 83972) has shown a protective effect against symptomatic recurrences [93,94]. Screening and treatment of ABU in these patient groups is therefore, not recommended. If these patient groups develop recurrent symptomatic UTI (see section 3.5) the potential protective effect of a spontaneously developed ABU against UTI must be considered before any treatment.
3.3.5.4.6. Patients with catheters in the urinary tract
Patients with indwelling or suprapubic catheters and nephrostomy tubes invariably become carriers of ABU, with antibiotic treatment showing no benefit [95]. This is also applicable for patients with ABU and indwelling ureteral stents [96]. Routine treatment of catheter-associated bacteriuria is not recommended. For detailed recommendations see section 3.8.
3.3.5.4.7. Patients with ABU subjected to catheter placements/exchanges
In patients subjected to uncomplicated placement/exchanges of indwelling urethral catheters ABU is not considered a risk factor and should not be screened or treated [97]. In patients subjected to placement/exchanges of nephrostomy tubes and indwelling ureteral stents, ABU is considered a risk factor for infectious complications [98].
3.3.5.4.8. Immuno-compromised and severely diseased patients, patients with candiduria
These patient groups have to be considered individually and the benefit of screening and treatment of ABU should be reviewed in each case. Patients with asymptomatic candiduria may, although not necessarily, have an underlying disorder or defect. Treatment of asymptomatic candiduria is not recommended [99].
3.3.5.5. Prior to urological surgery
In diagnostic and therapeutic procedures not entering the urinary tract, ABU is generally not considered as a risk factor, and screening and treatment are not considered necessary. On the other hand, in procedures entering the urinary tract and breaching the mucosa, particularly in endoscopic urological surgery, bacteriuria is a definite risk factor.
Two RCTs [100,101] and two prospective non-randomised studies [102,103] compared the effect of antibiotic treatment to no treatment before transurethral prostate or bladder tumour resections. Antibiotic treatment significantly reduced the number of post-operative symptomatic UTIs compared to no treatment in the meta-analysis of the two RCTs (average RR 0.20, 95% CI: 0.05 to 0.86; n=167). The rates of post-operative fever and septicaemia were also significantly lower in case of antibiotic treatment compared to no treatment in the two RCTs. One RCT including patients with spinal cord injury undergoing elective endoscopic urological surgeries found no significant difference in the rate of post-operative UTIs between single-dose or 3-5 days short term pre-operative antibiotic treatment of ABU [104].
A urine culture must therefore be taken prior to such interventions and in case of ABU, pre-operative treatment is recommended.
3.3.5.6. Prior to orthopaedic surgery
One RCT (n=471) and one multicentre cohort study (n=303) comparing the treatment of ABU with no treatment prior to orthopaedic surgery (hip arthroplasty/hemiarthroplasty or total knee arthroplasty) were identified [105,106]. Neither of the studies showed a beneficial effect of antibiotic treatment in terms of prosthetic joint infection (3.8% vs. 0% and 3.9% vs. 4.7%, respectively). The cohort study reported no significant difference in the rate of post-operative symptomatic UTI (0.65% vs. 2.7%) [106]. One further RCT investigated the efficacy of preoperative ABU treatment with fosfomycin-trometamol for prevention of early-periprosthetic joint infections (PJI) after hip hemiarthroplasty for fractures. Asymptomatic bacteriuria was not predictive of early-PJI (OR: 1.06, 95% CI: 0.33 - 3.38), and its treatment did not modify early-PJI incidence (OR: 1.03, 95% CI: 0.15-7.10) [107]. Furthermore, four additional meta-analyses did not find a benefit for preoperative screening or treatment of ABU prior to orthopaedic surgery [108-111]. Therefore, treatment of bacteriuria is not recommended prior to arthroplasty surgery.
3.3.5.7. Prior to cardiovascular surgery
One systematic review and meta-analysis including three retrospective non-randomised studies involving a total of 1,116 patients was identified [112]. The procedures performed were non-valvular coronary artery bypass grafting (42%), valvular replacements (51%), and thoracic aortic surgeries (7%). Preoperative treatment of ABU in 116 patients did not result in significant benefit regarding the rate of SSI compared to no treatment (12.9% vs. 8.2%, p =.0.086). A moderate heterogeneity was observed in the meta-analysis and preoperative treatment of ABU had no significant effect on the rate of infectious complications (OR: 1.38, 95% CI: 0.56 - 3.39). Due to the very low number, retrospective and non-randomised design of the included studies limited conclusions can be drawn from this. Further studies with appropriate design and sample size are needed to confirm these findings.
3.3.5.8. Pharmacological management
If the decision is taken to eradicate ABU, the treatment should be tailored according to the drug sensibility pattern of the pathogen identified. Treatment should be tailored and not empirical.
3.3.6. Follow-up
There are no studies focusing on follow-up after treatment of ABU.
3.3.7. Summary of evidence and recommendations for the management of ABU
| Summary of evidence | LE |
| Treatment of asymptomatic bacteriuria is not beneficial in the following conditions: |
|
| 3b |
| 1b |
| 1a |
| 1a |
| 2b |
| 1a |
| 1a |
| 1b |
| Treatment of asymptomatic bacteriuria is harmful in patients with recurrent urinary tract infections. | 1b |
| Treatment of asymptomatic bacteriuria is beneficial prior to urological procedures breaching the mucosa. | 1a |
| Treatment of asymptomatic bacteriuria in pregnant women was found to be beneficial by meta-analysis of the available evidence; however, most studies are old. A recent study reported lower rates of pyelonephritis in low-risk women. | 1a |
| Recommendations | Strength rating |
Do not screen or treat asymptomatic bacteriuria in the following conditions:
| Strong |
| Do not screen or treat asymptomatic bacteriuria in patients prior to cardiovascular surgeries. | Weak |
| Screen for and treat asymptomatic bacteriuria prior to urological procedures breaching the mucosa. | Strong |
| Screen for and treat asymptomatic bacteriuria in pregnant women with standard short course treatment or single dose fosfomycin trometamol. | Weak |
3.4. Cystitis
3.4.1. Introduction
Cystitis refers to inflammation of the bladder, typically characterised by symptoms such as urinary frequency, urgency, dysuria, suprapubic pain, and sometimes gross hematuria. It occurs without any signs or symptoms of systemic infection, such as fever or chills, in both men and women.
3.4.2. Epidemiology, aetiology and pathophysiology
Almost half of all women will experience at least one episode of cystitis during their lifetime. Nearly one in three women will have had at least one episode of cystitis by the age of 24 years [113]. Risk factors include sexual intercourse, use of spermicides, a new sexual partner, a mother with a history of cystitis and a history of cystitis during childhood. The majority of cases of cystitis are caused by E. coli.
3.4.3. Diagnostic evaluation
3.4.3.1. Clinical diagnosis
The diagnosis of cystitis can be made with a high probability based on a focused history of lower urinary tract symptoms (dysuria, frequency and urgency) and the absence of vaginal discharge [114,115]. In elderly women genitourinary symptoms are not necessarily related to cystitis [116,117].
3.4.3.2. Differential diagnosis
Cystitis should be differentiated from ABU, which is considered not to be an infection but rather a commensal colonisation, which should not be treated and therefore not screened for, except if it is considered a risk factor in clearly defined situations (see section 3.3).
3.4.3.3. Laboratory diagnosis
In patients presenting with typical symptoms of an cystitis urine analysis (i.e. urine culture, dip stick testing, etc.) leads only to a minimal increase in diagnostic accuracy [118]. However, if the diagnosis is unclear, dipstick analysis can increase the likelihood of an cystitis diagnosis [119,120]. Taking a urine culture is recommended in patients with atypical symptoms, as well as those who fail to respond to appropriate antimicrobial therapy [121,122].
3.4.3.4. Summary of evidence and recommendations for the diagnostic evaluation of cystitis
| Summary of evidence | LE |
| An accurate diagnosis of cystitis can be based on a focused history of lower urinary tract symptoms and the absence of vaginal discharge or irritation. | 2b |
| Recommendations | Strength rating |
Diagnose cystitis in women who have no other risk factors for systemic urinary tract infections based on:
| Strong |
| Use urine dipstick testing for diagnosis of acute cystitis. | Weak |
Urine cultures should be done in the following situations:
| Strong |
3.4.4. Disease management
3.4.4.1. Non-antibiotic treatments for the management of cystitis
3.4.4.1.1. Evidence Summary
In July 2024, an update on the role of nutraceuticals in managing cystitis was conducted, 995 abstracts were identified, retrieved and screened for relevance, with 44 ultimately selected for full-text review. This review resulted in the inclusion of twenty systematic reviews or guidelines based on systematic literature searches and 24 original publications for further analysis in the guidelines. Among the clinical trials, nine studies evaluated the efficacy of nutraceuticals in managing acute episodes of cystitis, while sixteen focused on recurrence prevention. Cranberry extract, either alone or in combination with other compounds, was the most extensively studied nutraceutical. The primary focus was determining which nutraceutical compounds effectively alleviate symptoms during the acute phase of cystitis and reduce recurrence rates in women with acute or recurrent symptomatic cystitis.
3.4.4.1.2. Management of acute cystitis
Treatment with cranberry products
The role of cranberry products in managing acute cystitis was evaluated in a systematic review that included three RCTs [123]. In two of those RCTs cranberry juice was used [124,125], while the third trial utilised encapsulated cranberry powder [126]. Within all three RCTs the Proanthocyanidins content varied significantly (from 7.5 to 224 mg) and the primary objective of the studies was not assessment of cranberry extract as an acute cystitis treatment. The risk of bias of the included studies was judged as moderate. Hence, the current evidence supporting or opposing the use of cranberry products for managing acute cystitis remains insufficient.
Treatment with D-mannose
The role of D-mannose in managing cystitis symptoms was investigated in a systematic review [127] that included seven studies, two of which were RCTs [128,129]. One RCT compared the use of D-mannose combined with cranberry and antibiotics to antibiotics alone [128], while the other compared D-mannose alone to antibiotics [129]. Among the non-RCTs, only one study evaluated D-mannose alone, with the remainder testing it in combination with other compounds [130]. Although this review suggests potential benefits of D-mannose in treating acute cystitis, current evidence remains insufficient to recommend it in this setting, due to the limited data for a detailed analysis of the effects of different doses of D-mannose or its impact when used alone versus in combination with other compounds. Moreover, most of the data originates from uncontrolled studies. Overall, the findings from this systematic review and meta-analysis are based on a very limited number of studies with small sample sizes.
A recent single-centre, randomised, double-blind, placebo-controlled trial examined the efficacy of D-mannose-based dietary supplement (D-mannose, citric acid, prebiotic fibers, Astragalus, and dandelion; DAPAD complex) for subjective (clinical resolution/response) and objective (midstream bacteriuria) outcomes in women with acute E. coli cystitis [131]. This study reported higher clinical and bacteriological resolution rates in the treatment group compared to placebo, with both groups receiving standard care and advice on increased fluid intake. However, despite these findings, due to study limitations and limited evidence, D-mannose cannot yet be recommended in this context.
Treatment with phytotherapeutics
A double-blind, parallel group, randomised, multicentre, non-inferiority phase III trial aimed to determine whether herbal therapy with Centaurii herba, Levistici radix, and Rosmarini folium (BNO 1045) is non-inferior to fosfomycin trometamol in treating acute lower cystitis [132]. Women aged 18-70 years with typical symptoms of newly diagnosed acute lower cystitis were randomised to BNO 1045 (n = 325) or fosfomycin trometamol (n = 334), with a corresponding matched placebo. The primary endpoint was the proportion of patients who received additional antibiotics to treat cystitis between days 1 and 38 ±3. Between days 1 and 38, 238 (83.5%) patients in the BNO 1045 group and 272 (89.8%) patients in the FT group received no additional antibiotics. At a 15% non-inferiority margin, BNO 1045 was non-inferior to FT in treating acute lower cystitis (non-AB rate difference: -6.26%; 95% CI -11.99 to -0.53%; 2-sided p = 0.0014). This study demonstrated the non-inferiority of this herbal combination compared to fosfomycin trometamol.
Another RCT evaluated the efficacy of a phytotherapeutics combination of L-Methionine accompanied by Hibiscus sabdariffa and Boswellia serrata for the treatment of acute episodes of cystitis in women affected by recurrent cystitis [133]. Forty-six patients were enrolled in the phytotherapeutics combination group (Group A), and 47 in the short-term antibiotic treatment (Group B). At the first follow-up (30 days), both groups showed a statistically significant improvement in quality-of-life scores as compared with baseline assessment [Group A: (QoL 94.3 VS 98.5 p < 0.001); Group B: (QoL 94.5 VS 98.7 p < 0.001)]. An improvement from baseline was also seen at the second follow-up evaluation after 3 months [Group A: (QoL 94.3 VS 99.1 p < 0.001); Group B: (QoL 94.5 VS 98.1 p < 0.001)]. At the second follow-up visit, a statistically significant difference in QoL was reported between the two groups (99.1 VS 98.1; p < 0.003) and a transition from cystitis to ABU was observed in twelve of 46 (26%) patients in Group A, while no patients in Group B demonstrated ABU (p = 0.007). It has been demonstrated that phytotherapeutics combination is able, in comparison to antibiotic treatment, to improve patients’ quality of life, reducing symptoms in acute setting and preventing the recurrences. Interestingly, a significantly higher proportion of patients in the phytotherapy group had ABU after three months.
A systematic review on herbal medicine for cystitis symptom control highlighted that combinations containing Centaurii herba, Levistici radix, Rosmarini folium, L-methionine, Hibiscus sabdariffa, and Boswellia serrata show promise for treating women with recurrent cystitis [134].
Treatment with xyloglucan, gelose, hibiscus and propolis
In a multicentre, randomised, parallel group, double-blind, phase IV study, the safety and efficacy of a combination containing xyloglucan-gelose-hibiscus-propolis were investigated as adjuvant therapy to first-line antimicrobials for treatment of cystitis in adults [135]. In this study the medical device (n = 20) or placebo
(n = 20) were administered orally in combination with an antimicrobial agent (e.g., ciprofloxacin) for five days, then alone for five days, then beginning on day 30 of the study for fifteen days per month for two months. Xyloglucan/gelose reduced positive urine cultures (defined as a bacterial count ≥103 CFU/mL) from 100% of patients at baseline to 0% at day eleven, with recurrence in three patients (15%) by day 76. Corresponding results with placebo were all patients had positive urine cultures at baseline reduced to 45% at day eleven, with recurrence in fourteen patients (70%) by day 76. Xyloglucan/gelose significantly reduced the frequency of urinary incontinence and urgency of micturition compared with placebo (both p < 0.05), with symptom resolution in all patients by day 90. These findings were supported by a systematic review and meta-analysis of studies testing this xyloglucan-containing preparation [136]. The primary endpoint was clinical or microbiological success, defined as the complete (cure) and/or non-complete (improvement) resolution of symptoms at the end of treatment, or microbiological resolutions. Three studies were included, recruiting a total of 178 patients. All three studies used placebo as comparator. A statistically significant difference was found in terms of clinical or microbiological resolution between the medical device and the comparator (three RCTs, 178 patients, OR: 0.13; 95% CI: 0.05–0.33; p < 0.0001). No clinically significant adverse effects have been reported.
3.4.4.1.3. Management of recurrent cystitis
Prophylaxis with probiotics (Lactobacillus spp.)
An RCT tested the prophylactic efficacy of vaginal probiotics alone and in combination with oral probiotics (containing lactic acid bacteria and bifidobacteria for oral use, and lactobacilli for vaginal use). The study found both the vaginal route alone and the combined oral and vaginal routes are effective in preventing symptomatic cystitis recurrence [137]. However, another systematic review reported no superiority of lactobacilli alone over placebo or cranberry monotherapy for recurrent cystitis, confirming earlier findings [138]. The current evidence is insufficient and of too low quality to provide specific recommendations on the administration route, optimal dosage, or duration of probiotic prophylaxis.
Prophylaxis with cranberry
In a separate RCT of 46 diabetic postmenopausal women, those randomised to receive 120 mg of a highly standardised cranberry extract phytosome showed a significant reduction in cystitis recurrence compared to placebo [139]. Another RCT with 172 adult women with a history of recurrent cystitis, comparing high-dose proanthocyanidins (240 mg) to placebo, reported that cranberry extract was associated with reduced cystitis and prolonged cystitis-free survival [140]. These studies underscore the importance of further research to determine the most effective concentration and formulation of cranberry for managing recurrent cystitis.
Regarding cranberry formulation, a systematic review indicated that cranberry juice, combined with increased fluid intake, may reduce cystitis rates and antibiotic use [141]. However, optimal dosing and treatment duration remain unclear.However, another systematic review and meta-analysis of eighteen RCTs and two non-RCTs, with moderate to low certainty, supports the use of cranberry products for symptom relief and reducing antibiotic usage [141]. This study also highlights the potential benefits of increased fluid intake in reducing cystitis rates, emphasising the advantage of cranberry in liquid form [141].
Prophylaxis with D-mannose
A systematic review and meta-analysis of eight studies (two using D-mannose alone and six using it in combination with other treatments) indicated that D-mannose may be protective against recurrent cystitis compared to placebo. Nonetheless, these findings must be interpreted cautiously due to the small number of studies, variation in study design (only two RCTs), quality issues, and overall limited sample size [142].
To determine whether D-mannose taken for six months reduces the proportion of women with recurrent cystitis experiencing a medically attended cystitis a 2-group (2g daily of D-mannose powder or matched volume of placebo powder), a double-blind randomised placebo-controlled trial was performed [143]. The primary outcome measure was the proportion of women experiencing at least one further episode of clinically suspected cystitis for which they contacted ambulatory care within six months of study entry. Secondary outcomes included symptom duration, antibiotic use, time to next medically attended cystitis, number of suspected episodes of cystitis, and cystitis-related hospital admissions. The proportion contacting ambulatory care with a clinically suspected cystitis was 150 of 294 (51.0%) in the D-mannose group and 161 of 289 (55.7%) in the placebo group (risk difference, −5%; 95% CI: −13% to 3%; p = 0.26). Estimates were similar in per protocol analyses, imputation analyses, and preplanned subgroups. There were no statistically significant differences in any secondary outcome measures. In this RCT, daily D-mannose did not reduce the proportion of women with recurrent cystitis in primary care who experienced a subsequent clinically suspected cystitis.
Prophylaxis with Xyloglucan, Hibiscus, and Propolis
Preclinical evidence supports the use of xyloglucan in managing recurrent cystitis by creating a barrier that prevents uropathogenic Escherichia coli from adhering to cell walls, demonstrated in models of intestinal and uroepithelial cells [136]. A systematic review and meta-analysis including three RCTs, with data on 178 patients, found that the combination of xyloglucan, hibiscus, and propolis was effective in preventing recurrent cystitis compared to placebo, showing high patient compliance and a reduction in antibiotic use [136]. Additionally, another review also highlighted the efficacy of this approach in managing recurrent cystitis [144].
Prophylaxis with Centaurii herba, Levistici radix, Rosmarini folium
An RCT involving 90 patients with cystitis compared antimicrobial therapy alone (control group) with antimicrobial therapy plus a combination of Centaurii herba, Levistici radix, and Rosmarini folium for three months (2 tablets, three times daily). The study found that the frequency of recurrent cystitis episodes was consistently lower in the intervention group than in the control group [145]. This combination has also been tested in pregnant women, showing no teratogenic, embryotoxic effects, or developmental defects in infants [146].
In the context of rising antimicrobial resistance, antibiotic-sparing strategies are increasingly essential for therapeutic and environmental benefits. Patients should be informed about these strategies, and urologists are encouraged to provide accurate information on the use of nutraceuticals in clinical practice, supported by a broad body of research and data.
3.4.4.1.4. Summary of evidence and recommendations for non-antibiotic management of cystitis
| Summary of evidence | LE |
| An RCT showed that Centaurii herba, Levistici radix, and Rosmarini folium alone are effective in relieving acute cystitis symptoms compared to fosfomycin trometamol. | 1b |
| An RCT demonstrated the efficacy of L-methionine combined with Hibiscus sabdariffa and Boswellia serrata in relieving acute cystitis symptoms, compared to fosfomycin trometamol. | 1b |
| A combination of xyloglucan, hibiscus, and propolis is effective in relieving acute cystitis symptoms and preventing recurrence. | 1a |
| Centaurii herba, Levistici radix, and Rosmarini folium are effective in preventing recurrence and reducing antibiotic use. | 1b |
| No superiority has been found for oral lactobacilli alone in preventing recurrent cys-titis compared to placebo or cranberry monotherapy. | 1a |
| Highly standardised cranberry extract phytosome and high-dose proanthocyanidins appear effective in preventing cystitis recurrence episodes. | 1b |
| There is contradictory evidence on the efficacy of D-mannose to reduce the number of cystitis episodes. | 2 |
| Recommendations | Strength rating |
| Advise female patients on the possibility of antibiotic-sparing approaches for the treatment and prevention of acute and recurrent cystitis. Patients should be fully informed on the level of evidence for the different approaches. | Strong |
| Use a combination of xyloglucan, hibiscus, and propolis, or Centaurii herba, Levistici radix, and Rosmarini folium to reduce recurrent cystitis episodes and reduce antibiotic use. | Weak |
3.4.4.2. Antibiotic treatment
Antimicrobial therapy is recommended because clinical success is significantly more likely in women treated with antimicrobials compared with placebo [147]. In non-geriatric patients, non-antibiotic therapy alone should be considered as an alternative to antibiotic treatment. A shared decision-making process with the patients is essential [148] .
The use of nonsteroidal anti-inflammatory drugs, such as ibuprofen in 67% [149], 65% [150], 53% [151], or diclofenac in 37% [152], and the use of phytotherapy like Uva Ursi in 64% [153] or BNO 1045 in 84% [132] resulted in a reduction of antibiotic therapy. Overall, across all studies, non-antibiotic therapy led to a 63% reduction in antibiotic use [154]. For geriatric patients, there is no evidence regarding symptomatic treatment with nonsteroidal anti-inflammatory drugs. Furthermore, nonsteroidal anti-inflammatory drugs are considered potentially inappropriate medications with associated risks in this age group [155].
When deciding on a therapy, the preferences of the patients should be appropriately considered. This is particularly true for primarily non-antibiotic treatment, which may involve accepting a higher symptom burden and increased complication rates, such as pyelonephritis.
The choice of antimicrobial therapy should be guided by [114]:
- spectrum and susceptibility patterns of the aetiological pathogens;
- efficacy for the particular indication in clinical studies;
- tolerability and adverse reactions;
- adverse ecological effects;
- costs;
- availability.
According to these principles and the available susceptibility patterns in Europe, oral treatment with fosfomycin trometamol 3 g single dose, pivmecillinam 400 mg three times a day for three to five days, and nitrofurantoin (e.g. nitrofurantoin monohydrate/macrocrystals 100 mg twice daily for five days), should be considered for first-line treatment, when available [156-159].
Alternative antimicrobials include trimethoprim alone or combined with a sulphonamide. Co-trimoxazole (160/800 mg twice daily for three days) or trimethoprim (200 mg twice daily for five days) should only be considered as drugs of first choice in areas with known resistance rates for E. coli of < 20% [160,161].
Aminopenicillins are no longer suitable for empirical therapy because of worldwide high E. coli resistance. Aminopenicillins in combination with a beta-lactamase inhibitor such as ampicillin/sulbactam or amoxicillin/clavulanic acid and oral cephalosporins are not recommended for empirical therapy due to ecological collateral damage, but may be used in selected cases [162,163].
Important note:
On March 11, 2019 the European Commission implemented stringent regulatory conditions regarding the use of fluoroquinolones due to their disabling and potentially long-lasting side effects [164]. This legally binding decision is applicable in all EU countries. National authorities have been urged to enforce this ruling and to take all appropriate measures to promote the correct use of this class of antibiotics. In cystitis, a fluoroquinolone should only be used when it is considered inappropriate to use other antibacterial agents that are commonly recommended for the treatment of these infections [164].
3.4.4.3. Cystitis with risk factors
3.4.4.3.1. Cystitis in pregnancy
Short courses of antimicrobial therapy can also be considered for treatment of cystitis in pregnancy [165], but not all antimicrobials are suitable during pregnancy. In general, penicillins, cephalosporins, fosfomycin, nitrofurantoin (not in case of glucose-6-phosphate dehydrogenase deficiency and during the end of pregnancy), trimethoprim (not in the first trimenon) and sulphonamides (not in the last trimenon), can be considered.
3.4.4.3.2. Renal insufficiency
In patients with renal insufficiency the choice of antimicrobials may be influenced by decreased renal excretion; however, most antimicrobials have a wide therapeutic index. No adjustment of dose is necessary until glomerular filtration rate (GFR) is < 20 mL/min, with the exception of antimicrobials with nephrotoxic potential, e.g. aminoglycosides. The combination of loop diuretics (e.g. furosemide) and a cephalosporin is nephrotoxic. Nitrofurantoin is contraindicated in patients with an estimated glomerular filtration rate (eGFR) of less than 30 ml/min/1.73m2 as accumulation of the drug leads to increased side effects as well as reduced urinary tract recovery, with the risk of treatment failure [166].
3.4.4.4. Cystitis in men
Cystitis in men without involvement of the prostate is uncommon. Therefore, treatment with antimicrobials penetrating into the prostate tissue is needed in males with symptoms of UTI. A treatment duration of at least seven days is recommended, preferably with trimethoprim-sulphamethoxazole or a fluoroquinolone if in accordance with susceptibility testing [167].
3.4.4.5. Summary of evidence and recommendations for antimicrobial therapy for cystitis
| Summary of evidence | LE |
| Clinical success for the treatment of cystitis is significantly more likely in women treated with antimicrobials than placebo. | 1b |
| The use of nonsteroidal anti-inflammatory drugs and phytotherapy, has resulted in a reduction of antibiotic therapy. Overall, across all studies, non-antibiotic therapy led to a 63% reduction in antibiotic use. | 1a |
| Aminopenicillins are no longer suitable for antimicrobial therapy in cystitis because of negative ecological effects, high resistance rates and their increased selection for extended spectrum beta-lactamase (ESBL)-producing bacteria. | 3 |
| Recommendations | Strength rating |
| Prescribe fosfomycin trometamol, pivmecillinam or nitrofurantoin as first-line treatment for cystitis in women. | Strong |
| Use non-antibiotic therapy options as an alternative to antibiotic treatment in non-geriatric patients. Shared decision-making with the patients is essential. | Strong |
| Do not use aminopenicillins or fluoroquinolones to treat cystitis. | Strong |
Table 3: Suggested regimens for antimicrobial therapy in cystitis
| Antimicrobial | Daily dose | Duration of therapy | Comments |
| First-line women | |||
| Fosfomycin trometamol | 3 g SD | 1 day | Recommended only in women with cystitis. |
| Nitrofurantoin macrocrystal | 50-100 mg four times a day | 5 days | |
| Nitrofurantoin monohydrate/macrocrystals | 100 mg b.i.d | 5 days | |
| Nitrofurantoin macrocrystal prolonged release | 100 mg b.i.d | 5 days | |
| Pivmecillinam | 400 mg t.i.d | 3-5 days | |
| Alternatives | |||
| Cephalosporins (e.g. cefadroxil) | 500 mg b.i.d | 3 days | Or comparable |
| If the local resistance pattern for E. coli is < 20% | |||
| Trimethoprim | 200 mg b.i.d | 5 days | Not in the first trimenon of pregnancy |
| Trimethoprim-sulfamethoxazole | 160/800 mg b.i.d | 3 days | Not in the last trimenon of pregnancy |
| Treatment in men | |||
| Trimethoprim-sulfamethoxazole | 160/800 mg b.i.d | 7 days | Restricted to men, fluoroquinolones can also be prescribed in accordance with local susceptibility testing. |
SD = single dose; b.i.d = twice daily; t.i.d = three times daily.
3.4.5. Follow-up
Routine post-treatment urinalysis or urine cultures in asymptomatic patients are not indicated [23]. In women whose symptoms do not resolve by end of treatment, and in those whose symptoms resolve but recur within two weeks, urine culture and antimicrobial susceptibility testing should be performed [168]. For therapy in this situation, one should assume that the infecting organism is not susceptible to the agent originally used. Retreatment with a seven-day regimen using another agent should be considered [168].
3.5. Recurrent cystitis
3.5.1. Introduction
Recurrent cystitis is defined by at least three episodes of cystitis/year or two episodes of cystitis in the last six months. Recurrent cystitis negatively impacts patient quality of life leading to a reduction in the quality of social and sexual relationships, self-esteem and capacity for work [169].
3.5.2. Diagnostic evaluation
Recurrent cystitis is common. Risk factors are outlined in Table 4. Initial diagnosis of recurrent cystitis should be confirmed by urine culture. An extensive routine workup including cystoscopy, imaging, etc. is not routinely recommended as the diagnostic yield is low [170]. However, it should be performed without delay in atypical cases, for example, if renal calculi, outflow obstruction, interstitial cystitis or urothelial cancer is suspected.
Table 4: Age-related associations of recurrent cystitis in women [73, 116, 171]
| Young and pre-menopausal women | Post-menopausal and elderly women |
Sexual intercourse Use of spermicide A new sexual partner A mother with a history of cystitis History of cystitis during childhood Blood group antigen secretory status | History of cystitis before menopause Urinary incontinence Atrophic vaginitis due to oestrogen deficiency Cystocele Increased post-void urine volume Blood group antigen secretory status Urine catheterisation and functional status deterioration in elderly institutionalised women |
3.5.3. Disease management and follow-up
Prevention of recurrent cystitis includes counselling regarding avoidance of risk factors, non-antimicrobial measures and antimicrobial prophylaxis [168,172]. These interventions should be attempted in this order. Any urological risk factor must be identified and treated. Significant residual urine should be treated optimally, including by CIC when judged to be appropriate.
3.5.3.1. Evidence Summary
A broad literature with cut-off of May 31st, 2021 identified 3,604 abstracts of which 361 were selected for full text review. In total 114 systematic reviews or guidelines based on systematic literature searches and 131 original publications were selected for further analysis. A further 18 relevant publications were identified from the references of the reviewed studies. Selected studies were assigned to one of nine subgroups based on the method of prevention. An updated search with cut-off date of 1st June 2022 identified a further 316 abstracts of which 25 were selected for further analysis. The evidence question addressed was: In women with recurrent symptomatic lower urinary tract infection, what interventions reduce the rate of recurrence?
3.5.3.2. Behavioural modifications
Women with recurrent cystitis should be counselled on avoidance of risks (e.g., insufficient drinking, habitual and post-coital delayed urination, wiping from front to back after defecation, douching and wearing occlusive underwear) before initiation of long-term prophylactic drug treatment, although there is limited evidence available regarding these approaches [173,174]. An open-label RCT found that additional fluid intake of 1.5 L a day in premenopausal women with recurrent cystitis who were low-volume drinkers (< 1.5 L a day) reduced the number of cystitis episodes and antibiotic usage over a twelve-month period [175].
3.5.3.3. Non-antimicrobial prophylaxis
3.5.3.3.1. Hormonal replacement
Based on the results of four meta-analyses topical oestrogen therapy (either as a creme or a pessary) shows a trend towards recurrent cystitis prevention [176-179]. All studies reported that application was superior compared to placebo but was inferior compared to antibiotics. Due to its pharmacokinetics vaginal admission has no systematic side effects, however local irritation and minor bleeding can occur. The use of oral oestrogens was not effective for recurrent cystitis prophylaxis compared to placebo, furthermore it was associated with an unfavourable systematic side effect profile. A single prospective, non-comparative study of 30 pre-menopausal women with recurrent cystitis on oral contraceptives reported a beneficial effect for additional topical oestrogen therapy [180].
3.5.3.3.2. Immunomodulation
A systematic review and meta-analysis evaluated the effectiveness of immunomodulation in preventing recurrent cystitis [181]. Fourteen comparative studies were included, with a total of 2,822 patients across five substances types (StroVac, OM-89, ExPEC4V, MV140, and Solco-Urovac). The pooled risk ratio from eight placebo-controlled studies on the percentage of patients remaining cystitis-free in the short-term (six to twelve months) was 1.52 (95% CI: 1.05–2.20), indicating that patients treated with immunomodulation were approximately 50% more likely to remain cystitis-free in the short-term in comparison to placebo. The number needed to treat (NNT) was 6.45 (95% CI: 2.80–64.80). However, there was substantial heterogeneity and a risk of publication bias. A subgroup analysis by substance type suggests that short-term effectiveness in reducing recurrent cystitis compared to placebo varies depending on the agent. Current evidence indicates no significant difference between OM-89, Solco-Urovac, and ExPEC4V for short-term cystitis prevention compared to placebo, as the risk ratios favoured the immunomodulatory agents but were not statistically significant. Data for MV140 compared to placebo show a risk ratio (RR) of 2.23 (95% CI: 1.43–3.47), the largest difference among all included studies, but this is from a single RCT of 146 participants. Three additional cohort studies comparing MV140 with antibiotic therapy demonstrated a significantly higher percentage of cystitis-free patients in the immunomodulatory group.
The review also concluded that cystitis immunomodulatory agents are generally safe, with serious adverse events (AEs) being rare and no reports of AEs leading to death. A pooled analysis found no significant difference in AE incidence rates between immunomodulatory agents and placebo. The most frequently reported AEs were headaches, gastrointestinal disturbances, and vaginitis. Immunomodulatory-specific side effects appear to be related to the agent used and method of administration.
Given the low certainty of the available data, the previous strong recommendation is downgraded to weak. The composition and mechanism of action of individual immunomodulatory agents are sufficiently different that pooled analyses of results from these disparate trials is likely to result in excessive noise. The panel would therefore recommend further intensive study of the most promising agents in larger, high quality randomised clinical trials using standardised definitions of recurrent cystitis and other outcome measure.
3.5.3.3.3. Prophylaxis with probiotics (Lactobacillus spp.)
Five meta-analyses with differing results and eleven relevant systematic reviews were identified [177,182-195]. Two meta-analyses reported significant positive effects for recurrent cystitis prevention with effective probiotics compared to placebo [186,188]. The contradictory results of the four meta-analyses are a result of the analysis of different Lactobacillus strains and different administration regimes, treatment durations, and patient populations. Most studies concluded that not all Lactobacillus strains are effective for vaginal flora restoration and recurrent cystitis prevention. The highest efficacy was shown with L. rhamnosus GR-1, L. reuteri B-54, L. reuteri RC-14, L. casei shirota, and L. crispatus CTV-05 [177,184,186,188]. Although meta-analyses including all known Lactobacilli strains did not show a significant treatment benefit [177,184,186,188], sensitivity analysis excluding studies using ineffective strains resulted in a positive treatment effect [186].
Of the eleven systematic reviews, seven concluded that prophylaxis with vaginal probiotics has a beneficial clinical impact for the prevention of recurrent cystitis [178,179,182,185,187,189-192,194]. The available data is too minimal or of low quality to allow the panel to make recommendations on the route of admission, optimal dosage, and treatment duration for probiotic prophylaxis.
3.5.3.3.4. Prophylaxis with cranberry
Seven meta-analyses and several systematic reviews were identified [177,196-201]. A Cochrane systematic review and meta-analysis found that when compared with placebo, water or not treatment, cranberry products did not significantly reduce the occurrence of symptomatic cystitis overall or in women with recurrent cystitis [196]. However, six subsequent meta-analyses concluded that consumption of cranberry-containing products may protect against cystitis in certain patient populations [177,197-201]. The differing outcomes across the meta-analyses can be contributed to the clinical and methodological heterogeneity of the included studies [202]. An RCT of 145 women randomised to high-dose vs. low-dose cranberry proanthocyanidin extract reported no significant reduction in the number of symptomatic cystitis episodes between the groups [203]. Although the efficacy of cranberry products remains unclear, the panel consensus is that clinicians may recommend them for recurrent cystitis prevention in women who are informed of the weak evidence base due to their favourable benefit to harm ratio. However, there is no clear clinical evidence regarding the appropriate dose and treatment duration.
3.5.3.3.5. Prophylaxis with D-mannose
A meta-analysis including one RCT, one randomised cross-over trial and one prospective cohort study analysed data on 390 patients and found that D-mannose was effective for recurrent cystitis prevention compared to placebo with comparable efficacy to antibiotic prophylaxis [142]. Another systematic review, concluded that D-mannose had a significant effect on cystitis, but that further studies were needed to confirm these findings [182]. A further systematic review including 695 patients reported that D-Mannose improved quality of life and significantly reduced recurrent cystitis in both catheter and non-catheter users and was effective in reducing the incidence of recurrent cystitis and prolonging cystitis-free periods [204]. However, a Cochrane systematic review including 719 patients was unable to determine if D-mannose when compared to no treatment, other supplements or antibiotics, significantly reduced the number of recurrent cystitis episodes [205]. The overall quality of the evidence was low.
3.5.3.3.6. Endovesical instillation
Endovesical instillations of hyaluronic acid (HA) and chondroitin sulphate (CS) have been used for glycosaminoglycan (GAG) layer replenishment in the treatment of interstitial cystitis, overactive bladder, radiation cystitis, and for prevention of recurrent cystitis [206]. A meta-analysis (n=143) based on two RCTs and two non-RCTs found significantly decreased cystitis rates per patient/year and significantly longer mean cystitis recurrence times for HA and HA-CS therapy compared to control treatment [207]. In addition, subgroup analysis of the two RCTs using HA-CS reported a significantly decreased cystitis rate per patient-year, significantly longer mean cystitis recurrence time and a significantly better pelvic pain and urgency/frequency (PUF) total score. However, 24-h urinary frequency measured as number of voids in three days were not significantly improved after therapy [207].
Another meta-analysis (n=800) including two RCTs and six non-RCTs found that when compared to control treatment HA, with or without CS, was associated with a significantly lower mean cystitis rate per patient-year and a significantly longer time to cystitis recurrence [208]. Furthermore, HA-CS therapy was associated with significantly greater mean reductions in PUF total and symptom scores and the percentage of patients with cystitis recurrence during follow-up was also lower [208].
As randomised controlled studies are available only for HA plus CS, the quality of evidence is higher for the combination than for HA alone.
3.5.3.3.7. Methenamine hippurate
A Cochrane review from 2012 based on thirteen studies, with high levels of heterogeneity, concluded that methenamine hippurate may be effective for preventing cystitis in patients without renal tract abnormalities, particularly when used for short-term prophylaxis [209]. A meta-analysis from 2021 based on six studies found that although studies showed a trend towards a benefit for methenamine hippurate in prevention of recurrent cystitis, there was no statistically significant difference between the efficacy of methenamine hippurate and any comparators [210]. A subsequent RCT including 240 women randomised (1:1) to receive once-daily low-dose antibiotic prophylaxis or twice-daily methenamine hippurate for twelve months reported that the incident rate of patient-reported symptomatic cystitis decreased to 1.38 episodes per person per year for the methenamine hippurate group vs. 0.89 episodes per person per year for the antibiotic group. The absolute difference was 0.49 confirming that methenamine hippurate was not inferior to antibiotic prophylaxis. The rate of adverse events was similar in both groups and a sustained benefit for both treatment arms was observed at six months follow-up [211,212].
3.5.3.4. Antimicrobials for preventing recurrent cystitis
3.5.3.4.1. Continuous low-dose antimicrobial prophylaxis and post-coital prophylaxis
Four meta-analyses and numerous systematic reviews and guidelines were identified [179,213-223]. All available meta-analyses conclude that antibiotic prophylaxis is the most effective approach against cystitis recurrences compared with placebo or no treatment [213-215]. Antimicrobials may be given as continuous low-dose prophylaxis for longer periods, or as post-coital prophylaxis. There is no significant difference in the efficacy of the two approaches. There is no consensus about the optimal duration of continuous antimicrobial prophylaxis, with studies reporting treatment duration of three to twelve months. After discontinuation of the drug, cystitis tends to re-occur, especially among those, who have had three or more infections annually. It is mandatory to offer both continuous low-dose antimicrobial and post-coital prophylaxis after counselling, and when behavioural modifications as well as non-antimicrobial measures have been unsuccessful.
Differences in outcomes between antibiotics did not reach statistical significance. The choice of agent should be based on the local resistance patterns. Regimens include nitrofurantoin 50 mg or 100 mg once daily, fosfomycin trometamol 3 g once in a week, trimethoprim 100 mg once daily and during pregnancy cephalexin 125 mg or 250 mg or cefaclor 250 mg once daily [168,224,225]. Post-coital prophylaxis should be considered in pregnant women with a history of frequent cystitis before onset of pregnancy, to reduce their risk of cystitis [226].
3.5.3.4.2. Self-diagnosis and self-treatment
In patients with good compliance, self-diagnosis and self-treatment with a short course regimen of an antimicrobial agent should be considered [227]. The choice of antimicrobials is the same as for sporadic acute cystitis (section 3.4.4.4).
3.5.4. Summary of evidence and recommendations for the diagnostic evaluation and treatment of recurrent cystitis
| Summary of evidence | LE |
| Extensive routine workup including cystoscopy, imaging, etc. has a low diagnostic yield for the diagnosis of recurrent cystitis. | 3 |
| Increased water intake is an effective antimicrobial-sparing strategy to prevent recurrent cystitis in premenopausal women at high risk for recurrence who drink low volumes (< 1.5 L) of fluid daily. | 3 |
| Vaginal oestrogen replacement has shown a trend towards preventing recurrent cystitis in post-menopausal women. | 1b |
| There is limited evidence to suggest that immunomodulatory agents are effective at reducing cystitis recurrence in adult female patients in the short-term. | 1a |
| Of the currently available immunomodulatory agents, OM-89 and MV140 are the most widely studied, with results for MV140 showing the most promising results. | 1a |
| Probiotics containing L. rhamnosus GR-1, L. reuteri B-54 and RC-14, L. casei shirota, or L. crispatus CTV-05 are effective for vaginal flora restoration and have shown a trend towards prevention of recurrent cystitis. | 1b |
| Current scientific evidence regarding the efficacy of cranberry remedies in association with increased water intake for treating of acute symptoms of cystitis and recurrence prevention. | 1a |
| An RCT demonstrated the efficacy of D-mannose alone to relieve acute symptoms in acute cystitis, when compared with cotrimoxazole. | 1b |
| Based on limited evidence intravesical GAG therapy can reduce the number of cystitis per patient per year, and prolong the time interval between recurrent cystitis episodes. | 2 |
| An RCT demonstrated the non-inferiority of twice-daily methenamine hippurate to daily antibiotic prophylaxis. | 1b |
| Both continuous low-dose antimicrobial prophylaxis and post-coital antimicrobial prophylaxis, have been shown to reduce the rate of recurrent cystitis. | 1b |
| A prospective cohort study showed that intermittent self-start therapy is effective, safe and economical in women with recurrent cystitis. | 2b |
| Recommendations | Strength rating |
| Diagnose recurrent cystitis by urine culture. | Strong |
| Do not perform an extensive routine workup (e.g., cystoscopy, full abdominal ultrasound) in women younger than 40 years of age with recurrent cystitis and no risk factors. | Weak |
| Advise pre-menopausal women regarding increased fluid intake as it might reduce the risk of recurrent cystitis. | Weak |
| Use vaginal oestrogen replacement in post-menopausal women to prevent recurrent cystitis. | Strong |
| Use immunomodulatory prophylaxis to reduce recurrent cystitis in women in the context of a well-regulated clinical trial. | Weak |
| Advise patients on the use of local or oral probiotics, containing strains of proven efficacy, for vaginal flora regeneration to prevent cystitis. | Weak |
| Advise patients on the use of cranberry products, favouring juice, for symptom relief in acute cystitis and to prevent recurrence; however, patients should be informed that the quality of evidence underpinning this is low with contradictory findings. | Weak |
| Use D-mannose to reduce recurrent cystitis episodes, but patients should be informed of the overall weak and contradictory evidence of its effectiveness. | Weak |
| Use methenamine hippurate to reduce recurrent cystitis episodes in women without abnormalities of the urinary tract. | Strong |
| Use endovesical instillations of hyaluronic acid or a combination of hyaluronic acid and chondroitin sulphate to prevent recurrent cystitis in patients where less invasive preventive approaches have been unsuccessful. Patients should be informed that further studies are needed to confirm the results of initial trials. | Weak |
| Use continuous or post-coital antimicrobial prophylaxis to prevent recurrent cystitis when non-antimicrobial interventions have failed. Counsel patients regarding possible side effects. | Strong |
| For patients with good compliance, self-administered short-term antimicrobial therapy should be considered. | Strong |
3.6. Pyelonephritis
3.6.1. Diagnostic evaluation
3.6.1.1. Clinical diagnosis
Pyelonephritis is suggested by fever (> 38°C), chills, flank pain, nausea, vomiting, or costovertebral angle tenderness, with or without the typical symptoms of cystitis [228]. Pregnant women with acute pyelonephritis need special attention, as this kind of infection may not only have an adverse effect on the mother with anaemia, renal and respiratory insufficiency, but also on the unborn child with more frequent pre-term labour and birth [229].
3.6.1.2. Differential diagnosis
It is vital to differentiate as soon as possible between pyelonephritis with or without risk factors, such as obstructive pyelonephritis, as the latter can rapidly lead to urosepsis. This differential diagnosis should be made by the appropriate imaging technique (see section 3.6.1.4).
3.6.1.3. Laboratory diagnosis
Urinalysis including the assessment of white and red blood cells and nitrite, is recommended for routine diagnosis [230]. In addition, urine culture and antimicrobial susceptibility testing should be performed in all cases of pyelonephritis.
3.6.1.4. Imaging diagnosis
Evaluation of the upper urinary tract with ultrasound (US) should be performed to rule out urinary tract obstruction or renal stone disease in patients with a history of urolithiasis, renal function disturbances or a high urine pH [231]. Additional investigations, such as a contrast enhanced computed tomography (CT) scan, or excretory urography should be considered if the patient remains febrile after 72 hours of treatment, or immediately if there is deterioration in clinical status [231]. For diagnosis of complicating factors in pregnant women, US or magnetic resonance imaging (MRI) should be used preferentially to avoid radiation risk to the foetus [231].
3.6.2. Summary of evidence and recommendations for the diagnostic evaluation of pyelonephritis
| Summary of evidence | LE |
| Urine culture and antimicrobial susceptibility testing should be performed in all cases of pyelonephritis in addition to urinalysis. | 4 |
| A prospective observational cohort study found that radiologic imaging can selectively be applied in adults with systemic UTI without loss of clinically relevant information by using a simple clinical prediction rule. | 2b |
| Additional imaging investigations, such as an unenhanced helical computed tomography should be done if the patient remains febrile after 48-72 hours of treatment or in patients with suspected complications e.g. sepsis. | 4 |
| Recommendations | Strength rating |
| Perform urinalysis (e.g. using the dipstick method), including the assessment of white and red blood cells and nitrite, for routine diagnosis. | Strong |
| Perform urine culture and antimicrobial susceptibility testing in patients with pyelonephritis. | Strong |
| Perform imaging of the urinary tract to exclude urgent urological disorders. | Strong |
3.6.3. Disease management
3.6.3.1. Outpatient treatment
Fluoroquinolones and cephalosporines are the only antimicrobial agents that can be recommended for oral empirical treatment of pyelonephritis [232]. However, oral cephalosporines achieve significantly lower blood and urinary concentrations than intravenous cephalosporines. Other agents such as nitrofurantoin, oral fosfomycin, and pivmecillinam should be avoided as there is insufficient data regarding their efficacy [233]. In the setting of fluoroquinolone hypersensitivity or known resistance, other acceptable choices include trimethoprim-sulfamethoxazole (160/800 mg) or an oral beta-lactam, if the uropathogen is known to be susceptible. If such agents are used in the absence of antimicrobial susceptibility results, an initial intravenous dose of a long-acting parenteral antimicrobial (e.g. ceftriaxone) should be administered. A short outpatient antibiotic course of treatment, for acute pyelonephritis, has been shown to be equivalent to longer durations of therapy in terms of clinical and microbiological success. However, this is associated with a higher recurrence rate of infection within four to six weeks and needs to be tailored to local policies and resistance patterns [234].
3.6.3.2. Inpatient treatment
Patients with pyelonephritis requiring hospitalisation should be treated initially with an intravenous antimicrobial regimen e.g. a fluoroquinolone, an aminoglycoside (with or without ampicillin), or an extended-spectrum cephalosporin or penicillin [235]. Ceftolozane/tazobactam achieved a clinical response rate of over 90% in patients with pyelonephritis [236,237]. It also demonstrated significantly higher composite cure rates than levofloxacin among levofloxacin-resistant pathogens [238]. Ceftazidime-avibactam combination has been shown to be effective for treating ceftazidime-resistant Enterobacterales and Pseudomonas aeruginosa UTIs [239].
Novel antimicrobial agents include imipenem/cilastatin, cefiderocol, meropenem-vaborbactam and plazomicin. Imipenem/cilastatin has been investigated in a phase 2 randomised trial and showed good clinical response rates [240]. Cefatazidime-avibactam and doripenem showed similar efficacy against ceftazidime non-susceptible pathogens and may offer an alternative to carbapenems in this setting [241]. Meropenem-vaborbactam has been shown to be non-inferior to piperacillin-tazobactam in a phase 3 RCT [242]. It was also effective for treating carbapenem-resistant Enterobacterales with cure rates of 65% compared to best available treatment [243]. Once daily plazomicin was non-inferior to meropenem for the treatment of complicated UTIs and acute pyelonephritis caused by Enterobacterales, including multidrug-resistant strains [244]. Cefiderocol was non-inferior to imipenem/cilastatin for the treatment of complicated UTI in people with multidrug-resistant Gram-negative infections in a phase 2 RCT [245].
Carbapenems and novel broad spectrum antimicrobial agents should only be considered in patients with early culture results indicating the presence of multi-drug resistant organisms. The choice between these agents should be based on local resistance patterns and optimised on the basis of drug susceptibility results. In patients presenting with signs of urosepsis, empiric antimicrobial coverage for ESBL-producing organisms is warranted [246]. Patients initially treated with parenteral therapy who improve clinically and can tolerate oral fluids may transition to oral antimicrobial therapy [247].
3.6.3.3. Summary of evidence and recommendations for the treatment of pyelonephritis
| Summary of evidence | LE |
| Fluoroquinolones and cephalosporines are the only microbial agents that can be recommended for oral empirical treatment of pyelonephritis. | 1b |
| Intravenous antimicrobial regimens for pyelonephritis may include a fluoroquinolone, an aminoglycoside (with or without ampicillin), or an extended-spectrum cephalosporin or penicillin. | 1b |
| Carbapenems should only be considered in patients with early culture results indicating the presence of multi-drug resistant organisms. | 4 |
| The appropriate antimicrobial should be chosen based on local resistance patterns and optimised on the basis of drug susceptibility results. | 3 |
| Recommendations | Strength rating |
| Treat patients with pyelonephritis not requiring hospitalisation with short course fluoroquinolones as first-line treatment. | Strong |
| Treat patients with pyelonephritis requiring hospitalisation with an intravenous antimicrobial regimen initially. | Strong |
| Switch patients initially treated with parenteral therapy, who improve clinically and can tolerate oral fluids, to oral antimicrobial therapy. | Strong |
| Do not use nitrofurantoin, oral fosfomycin, and pivmecillinam to treat pyelonephritis. | Strong |
Table 5: Suggested regimens for empirical oral antimicrobial therapy in pyelonephritis
| Antimicrobial | Daily dose | Duration of therapy | Comments |
| Ciprofloxacin | 500-750 mg b.i.d | 7 days | Fluoroquinolone resistance should be less than 10%. |
| Levofloxacin | Standard dosage: 500 mg oral q.d High dosage: 500 mg oral b.i.d | 5 days | |
| Trimethoprim sulfamethoxazol | 160/800 mg b.i.d | 14 days | If such agents are used empirically, an initial intravenous dose of a long-acting parenteral antimicrobial (e.g. ceftriaxone) should be administered. |
| Cefpodoxime | 200 mg b.i.d | 10 days | |
| Ceftibuten | 400 mg q.d | 10 days |
b.i.d = twice daily; q.d = every day.
Table 6: Suggested regimens for empirical parenteral antimicrobial therapy in pyelonephritis
| Antimicrobials | Daily dose | Comments |
| First-line treatment | ||
| Ciprofloxacin | 400 mg b.i.d | |
| Levofloxacin | Standard dosage: 500 mg oral q.d High dosage: | |
| Cefotaxime | 2 g t.i.d | Not studied as monotherapy in acute pyelonephritis. |
| Ceftriaxone | Standard dosage: 2 g iv q.d High dosage: 2 g iv b.i.d | Lower dose studied, but higher dose recommended. |
| Second-line treatment | ||
| Cefepime | Standard dosage: 1 g iv t.i.d or 2 g iv b.i.d High dosage: 2 g iv t.i.d | Lower dose studied, but higher dose recommended. |
| Piperacillin/tazobactam | Standard dosage: 4.5 g t.i.d High dosage: 4.5 g q.i.d prolonged infusion | |
| Gentamicin | 6-7 mg/kg q.d | Not studied as monotherapy in acute uncomplicated pyelonephritis. |
| Amikacin | 25-30 mg/kg q.d | |
| Last-line alternatives | ||
| Imipenem/cilastatin | Standard dosage: 0.5 g iv q.i.d over 30 minutes High dosage: 1 g iv q.i.d over 30 minutes | Consider only in patients with early culture results indicating the presence of multi-drug resistant organisms. |
| Meropenem | 1 g t.i.d | |
| Ceftolozane/tazobactam | 1.5 g t.i.d | |
| Ceftazidime/avibactam | 2.5 g t.i.d | |
| Cefiderocol | 2 g t.i.d | |
| Meropenem-vaborbactam | 2 g t.i.d | |
| Plazomicin | 15 mg/kg o.d | |
b.i.d = twice daily; t.i.d = three times daily; q.d = every day; o.d = once daily.
In pregnant women with pyelonephritis, outpatient management with appropriate parenteral antimicrobials may also be considered, provided symptoms are mild and close follow-up is feasible [248,249]. In more severe cases of pyelonephritis, hospitalisation and supportive care are usually required. After clinical improvement, parenteral therapy can also be switched to oral therapy for a total treatment duration of seven to ten days. In men with febrile UTI, pyelonephritis, or recurrent infection, or whenever a complicating factor is suspected, a minimum treatment duration of two weeks is recommended, preferably with a fluoroquinolone since prostatic involvement is frequent [250].
3.6.4. Follow-up
Post-treatment urinalysis or urine cultures in asymptomatic patients post-therapy are not indicated.
3.7. Systemic urinary tract infections
3.7.1. Introduction
Systemic UTIs are infections that originate from various organs of the urinary tract. In contrast to localised UTIs (i.e., cystitis), these infections present typically with systemic signs and symptoms of infection (see Table 1). Local signs and symptoms of infection may also be present. Examples of systemic UTI include pyelonephritis, acute prostatitis, and urosepsis. Catheter-associated UTIs might present as localised as well as systemic infections.
3.7.2. Diagnostic evaluation
3.7.2.1. Clinical presentation
Systemic urinary tract infection should be suspected in patients presenting with dysuria, urinary frequency, urgency, or suprapubic pain, accompanied by fever, chills, flank pain, or pelvic/perineal pain or when patients appear clinically unwell. Systemic UTI may also be considered in patients with unexplained fever or sepsis. Evaluation should include a thorough clinical assessment to rule out other potential causes of illness. Physical examination should assess for costovertebral angle tenderness and abdominal or suprapubic tenderness. In females, a pelvic examination may be necessary, particularly when symptoms do not clearly suggest a UTI, to evaluate for cervical motion tenderness or uterine tenderness, which could indicate pelvic inflammatory or sexual transmitted disease. In biological men presenting with pelvic or perineal pain, a digital rectal examination is essential to assess for tenderness or swelling of the prostate, which may suggest acute prostatitis.
3.7.2.2. Urinalysis and urine culture
For patients with suspected systemic UTI, urine should be collected for both urinalysis and culture with antimicrobial susceptibility testing.
3.7.2.3. Pregnancy testing
Pregnancy testing is recommended for women of childbearing potential when pregnancy cannot be reasonably ruled out based solely on medical history.
3.7.2.4. Routine blood tests and blood cultures
Routine blood tests should always be considered in patients presenting with systemic signs and symptoms of infection. Blood cultures are indicated for patients presenting with sepsis or severe illness or when Staphylococcus aureus is isolated from the urine. S. aureus in urine is a “marker” of S. aureus blood stream infections (SABSI). Urine positive SABSI have a higher mortality than those without bacteriuria [251] as well as higher rates of recurrence after antibiotic cessation [252].
3.7.2.5. Imaging
In patients with systemic UTIs, the primary goal of imaging is to identify underlying conditions that may delay therapeutic response or require intervention (e.g. urinary retention, hydronephrosis, etc.) as well as to diagnose potential complications of infection (e.g. renal or perinephric abscess, prostatic abscess). Initial imaging should be performed by ultrasound. In case of hydronephrosis or if obstruction of the upper urinary tract is suspected, cross-sectional imaging (CT, MRI) should be carried out. In severe ill patients and in patients exhibiting persistent clinical symptoms despite 48 to 72 hours of appropriate antimicrobial therapy cross-sectional imaging (CT, MRI) should be performed as well [253,254].
3.7.3. General principles of systemic UTI treatment
Empiric antimicrobial therapy should be initiated without delay, with subsequent modifications based on antimicrobial susceptibility testing results. Identified or suspected anatomical abnormalities should be thoroughly evaluated and managed as indicated.
3.7.3.1. Choice of antimicrobials
3.7.3.1.1. Empiric antimicrobial therapy
Empiric therapy for systemic UTIs should consider illness severity, resistance risk factors and patient-specific factors [233]. Treatment selection depends on prior urinary isolate susceptibility, patient characteristics (e.g., allergies, tolerability, prior antimicrobial use), local resistance patterns and drug-related factors such as toxicity, interactions, availability, and costs. Upon identifying the pathogen’s susceptibility profile, the empiric regimen should be adjusted accordingly. Evidence for different systemic UTI regimens is limited, with few options formally evaluated [255,256].
3.7.3.1.2. Critical illness and/or urinary tract obstruction
In critically ill patients with systemic UTIs, patients worsening on current therapy or patients with suspected urinary tract obstruction, broad-spectrum antimicrobials are recommended. A carbapenem (e.g. imipenem (500 mg IV every six hours) or meropenem (1 g iv every eight hours) is advised to cover ESBL-producing organisms and Pseudomonas aeruginosa. Vancomycin or alternatives (daptomycin, linezolid) should be added for MRSA coverage. Broad-spectrum coverage is crucial in critically ill or obstructed cases due to high adverse outcome risks and increasing multidrug-resistant pathogens. If cultures confirm susceptibility, regimens should be narrowed. Advanced options like beta-lactamase-inhibitor combinations, cefiderocol, plazomicin, and parenteral fosfomycin, where available, target some ESBL-producing Enterobacterales and certain multidrug-resistant Pseudomonas aeruginosa strains [239,242,257-259]; however, use is reserved for highly resistant cases, given cost, antibiotic stewardship concerns, and limited data. Urine culture and susceptibility results should guide both confirmation and refinement of therapy, prioritising narrow-spectrum agents when possible.
3.7.3.1.3. Outpatients
Outpatient treatment may be appropriate for patients with acute systemic UTIs of mild to moderate severity who can reliably take oral medications. The choice of empiric antimicrobials should consider the risk of multidrug-resistant organisms, particularly ESBL producers. Fluoroquinolones are a key option, offering broad activity, including against Pseudomonas aeruginosa, and high urinary concentrations. Despite rising resistance rates, fluoroquinolones, when suitable, remain effective; ciprofloxacin and levofloxacin are preferred.
3.7.3.1.4. Directed antimicrobial therapy
Regimen selection
Urine culture and antimicrobial susceptibility results should guide tailoring of the antimicrobial regimen. Broad-spectrum empiric treatments can often be replaced by narrower-spectrum agents. Patients initially on parenteral therapy may transition to oral agents once symptoms improve, provided culture results support the switch.
Duration
Antimicrobial therapy duration generally ranges from five to ten days, based on clinical response and selected agent. In general, among hospitalised patients with bloodstream infection, antibiotic treatment for seven days is noninferior to treatment for fourteen days [260]. For patients with symptomatic improvement within 48–72 hours, recommended durations are five to seven days for fluoroquinolones, seven to ten days for trimethoprim-sulfamethoxazole [235] and seven to ten days for beta-lactams. If clinical response is slow (no improvement within 48–72 hours), further evaluation for complications or anatomical abnormalities is advised [247,261]. Extended therapy may be needed for persistent infection sources, such as a non-removable stone. Routine extension of therapy duration is unnecessary in cases of uncomplicated bacteremia without complicating factors, as bacteremia alone does not worsen prognosis, except for S. aureus bacteremia, addressed separately. Systematic reviews and meta-analyses show similar cure rates with shorter courses (≤ seven days) for systemic UTIs, mostly based on fluoroquinolone trials comparing five- or seven-day regimens to longer treatments [262-264]. However, in males with systemic UTIs seven days of antibiotic treatment is inferior to fourteen days [264,265]. Limited data exist for other antibiotics, but beta-lactam studies, primarily with older agents, showed no clear benefit from extending treatment beyond ten days [261,266].
3.7.3.1.5. Addressing risk factors
In addition to antimicrobial therapy, urinary obstruction should be assessed and managed if present. Patients with anatomical or functional urinary tract abnormalities (e.g., neurogenic lower urinary tract dysfunction, indwelling transurethral catheters, nephrostomy tubes, ureteral tents) may need additional interventions. Effective treatment may require addressing these underlying conditions, as antimicrobials alone may be insufficient [267].
3.7.3.1.6. Follow-up
Symptoms should improve quickly with effective antimicrobial therapy. Outpatients with pyelonephritis require follow-up, either in person or by phone, within 48–72 hours. Further evaluation is advised for patients with worsening symptoms post-initiation of antimicrobial therapy, persistent symptoms after 48–72 hours of appropriate therapy, or recurrence within weeks. This may include abdominal or pelvic imaging (typically cross-sectional imaging) to identify factors delaying recovery. Urine culture and antimicrobial susceptibility testing should also be repeated, and therapy adjusted as needed. For those presenting with haematuria, a follow-up urinalysis is recommended several weeks post-treatment to check for persistence.
3.7.4. Summary of evidence and recommendations for the treatment of systemic UTIs
| Summary of evidence | LE |
| Patients with a UTI with systemic symptoms requiring hospitalisation should be initially treated with an intravenous antimicrobial regimen chosen based on local resistance data and previous urine culture results from the patient. The regimen should be tailored on the basis of susceptibility result. | 1b |
| If the prevalence of fluoroquinolone resistance is thought to be < 10% and the patient has contraindications for third generation cephalosporins or an aminoglycoside, ciprofloxacin can be prescribed as an empirical treatment. | 2 |
| In the event of hypersensitivity to penicillin, a cephalosporins can still be prescribed, unless the patient has had systemic anaphylaxis in the past. | 2 |
| In patients with a systemic UTI, empirical treatment should cover ESBL if there is an increased likelihood of ESBL infection based on prevalence in the community, earlier urine cultures and prior antimicrobial exposure of the patient. | 2 |
| Recommendations | Strength rating |
Use the following antimicrobials as empirical intravenous treatment for systemic UTI:
| Strong |
Use ciprofloxacin provided:
| Strong |
| Do not use ciprofloxacin and other fluoroquinolones for the empirical treatment of systemic UTI in patients from urology departments or when patients have used fluoroquinolones in the last six months. | Strong |
| Manage any urological abnormality and/or underlying complicating factors. | Strong |
3.8. Catheter-associated UTIs
3.8.1. Introduction
Catheter-associated UTI refers to UTIs occurring in a person whose urinary tract is currently catheterised or has been catheterised within the past 48 hours. The urinary catheter literature is problematic as many published studies use the term CA-bacteriuria without providing information on what proportion are CA-ABU and CA-UTI, and some studies use the term CA-UTI when referring to CA-ABU or CA-bacteriuria [28].
3.8.2. Epidemiology, aetiology and pathophysiology
Catheter-associated UTIs are the leading cause of secondary healthcare-associated bacteraemia. Approximately 20% of hospital-acquired bacteraemias arise from the urinary tract, and the mortality associated with this condition is approximately 10% [268]. A multistate point-prevalence survey of 11,282 patients across 183 hospitals reported that UTI accounted for 12.9% of healthcare acquired infections [269]. The incidence of bacteriuria associated with indwelling catheterisation is 3-8% per day [270-274]. The duration of catheterisation is the most important risk factor for the development of a CA-UTI [275,276]. A systematic review and meta-analysis reported an average CA-UTI incidence of 13.79/1000 hospitalised patients with a prevalence of 9.33% [277]. This study also demonstrated that patients at high risk for CA-UTI were female, had a prolonged duration of catheterisation, had diabetes and had longer hospital and intensive care unit (ICU) stays [277].
Urinary catheterisation perturbs host defence mechanisms and provides easier access of uropathogens to the bladder. Indwelling urinary catheters facilitate colonisation with uropathogens by providing a surface for the attachment of host cell binding receptors recognised by bacterial adhesins, thus enhancing microbial adhesion. In addition, the uroepithelial mucosa is damaged, exposing new binding sites for bacterial adhesins, and residual urine in the bladder is increased through pooling below the catheter bulb [278]. Catheter-associated UTIs are often polymicrobial and caused by multiple-drug resistant uropathogens.
3.8.3. Diagnostic evaluation
3.8.3.1. Clinical diagnosis
Signs and systemic symptoms compatible with CA-UTI include new onset or worsening of fever, rigors, altered mental status, malaise, or lethargy with no other identified cause, flank pain, costovertebral angle tenderness, acute haematuria, pelvic discomfort and in those whose catheters have been removed dysuria, urgent or frequent urination and suprapubic pain or tenderness [279]. In the catheterised patient, the presence or absence of odorous or cloudy urine alone should not be used to differentiate CA-ABU from CA-UTI [28,279].
3.8.3.2. Laboratory diagnosis
Microbiologically, CA-UTI is defined by microbial growth of ≥ 103 cfu/mL of one or more bacterial species in a single catheter urine specimen or in a mid-stream voided urine specimen from a patient whose urethral, suprapubic, or condom catheter has been removed within the previous 48 hours [28]. In catheterised patients, pyuria is not diagnostic for CA-UTI. The presence, absence, or degree of pyuria should not be used to differentiate CA-ABU from CA-UTI. Pyuria accompanying CA-ABU should not be interpreted as an indication for antimicrobial treatment. The absence of pyuria in a symptomatic patient suggests a diagnosis other than CA-UTI [28].
3.8.3.3. Summary of evidence table and recommendations for diagnostic evaluation of CA-UTI
| Summary of evidence | LE |
| Patients with indwelling or suprapubic catheters become carriers of ABU, with antibiotic treatment showing no benefit. | 1a |
| In the catheterised patient, the presence or absence of odorous or cloudy urine alone should not be used to differentiate CA-ABU from CA-UTI. | 2 |
| Microbiologically, CA-UTI is defined by microbial growth of ≥ 103 cfu/mL of one or more bacterial species in a single catheter urine specimen or in a mid-stream voided urine specimen from a patient whose catheter has been removed within the previous 48 hours. | 3 |
| Recommendations | Strength rating |
| Do not carry out routine urine culture in asymptomatic catheterised patients. | Strong |
| Do not use pyuria as sole indicator for catheter-associated UTI. | Strong |
| Do not use the presence or absence of odorous or cloudy urine alone to differentiate catheter-associated asymptomatic bacteriuria from catheter-associated UTI. | Strong |
3.8.4. Disease management
3.8.4.1. Limiting catheterisation and appropriate catheter discontinuation
Indwelling catheters should be placed only when they are clinically indicated; for example, for management of urinary retention or where strict monitoring of fluid balance is required. Catheter restriction protocols are an important part of multi-modal interventions to reduce CA-UTI rates. Nurse-driven protocols in hospitals as well as community based multi-modal targeted infection programs have been proven to reduce CA-UTI rates [280,281]. Adjunctive devices such as electronic reminder systems have also been shown to assist in prompt catheter removal in hospital settings (including non-ICU). A systematic review of nineteen different interventions to reduce UTI (including catheter discontinuation and limiting catheterisation), in nursing home patients reported successful CA-UTI reduction and reduced catheter usage [282]. Another report of over 2,800 patients on a surgical oncology unit found that increasing catheter bundle compliance resulted in a significant reduction in CA-UTI rates [283].
3.8.4.2. Urethral cleaning and chlorhexidine bathing
A network meta-analysis of 33 studies (6,490 patients) found no difference in the incidence of CA-UTI comparing the different urethral cleaning methods vs. disinfection [284]. The efficacy of chlorhexidine baths (either using 2% chlorhexidine-impregnated cloths or 4% chlorhexidine-based soap) in reducing CA-UTI is debatable. In an RCT of 10,783 ICU patients, no difference in CA-UTI rates were reported between chlorhexidine and control bathing groups [285]. However, a systematic review of fifteen studies involving only ICU patients reported that daily chlorhexidine bathing was associated with a significant reduction in CA-UTI (RR 0.68) [286].
3.8.4.3. Alternatives to indwelling urethral catheterisation
Alternatives include intermittent urethral catheterisation (IC) or suprapubic catheterisation. In a systematic review of patients undergoing gynaecological surgery, indwelling catheters were associated with higher rates of symptomatic UTIs compared to IC [287]. A further meta-analysis of postpartum women reported no difference in the incidence of UTI after labour between continuous catheterisation and IC [287]. A prospective cohort study of nursing home residents found that residents with a suprapubic catheter had fewer CA-UTIs and where hospitalised less, but were more likely to be colonised with multi drug resistant organisms [288].
A Cochrane review found insufficient evidence to assess the value of different policies for replacing long-term urinary catheters on patient outcomes [97]. Another Cochrane review investigating the role of urethral (indwelling or intermittent) vs. suprapubic catheterisation in the short-term found inconclusive evidence of an effect on UTI rates [289]. For patients with NLUTD, a further systematic review found no randomised or quasi-randomised controlled trials and therefore no conclusions regarding the use of the different types of catheters could be made [290]. Therefore, based on the available literature, while there are some limited studies showing a benefit of IC or suprapubic catheterisation over urethral catheterisation for CA-UTI rates, there is insufficient evidence to recommend those approaches routinely [291].
3.8.4.4. Impregnated or coated catheters
Hydrophilic coated catheters have been found to be beneficial for reducing CA-UTI rates. A meta-analysis of seven studies investigating RCTs comparing hydrophilic coated to PVC (standard) catheters for IC found a statistically lower risk ratio (0.84) for the frequency of UTI in the hydrophilic catheter group [292]. A systematic review and practice policy statements on UTI prevention in patients with spina bifida recommended the use of single-use and hydrophilic catheters for IC [293].
Silver-alloy-impregnated catheters have not been associated with reduced CA-UTI rates. A small RCT of 54 ICU patients showed no significant difference in UTI rates between the silver-alloy impregnated group and the standard silicone foley catheter group [294]. In a cohort study of patients undergoing suprapubic catheter placement at the time of pelvic organ prolapsed surgery, a 5% difference in UTI rate at six weeks was noted, although this was not significant [295]. A systematic review of 26 trials (12,422 patients) reported that silver alloy-coated catheters were not associated with a statistically significant reduction in CA-UTI and were considerably more expensive [296]. However, the same study found that nitrofurazone-impregnated catheters reduce the risk of symptomatic CA-UTI; however, this was borderline significant (RR 0.84, 95% CI: 0.71 to 0.99) [296]. A more recent RCT (214 patients) evaluating the use of nitrofurazone-infused catheters post-renal transplant found no benefit for their use [297]. Additionally, another RCT showed no benefit for the use of silver-alloy-coated indwelling catheters for reduction of UTI in 489 patients with spinal cord injury [298].
From a microbiological perspective, there may be a difference in organisms causing CA-UTI from urethral and suprapubic catheters and therefore urine culture results are important to guide therapy [291].
3.8.4.5. Antibiotic prophylaxis for catheter removal or insertion
The issue of whether antibiotic prophylaxis reduce the rate of symptomatic UTI in adults following indwelling bladder catheter removal has been the subject of multiple RCTs. A review and meta-analysis identified seven RCTs with 1,520 participants. Meta-analysis showed overall benefit for use of prophylaxis RR (95%CI) = 0.45 (0.28-0.72); ARR 5.8% (from 10.5% to 4.7%) with a number needed to treat (NNT) of 17 [214]. Results for individual trials were inconsistent with five trials including the possibility of no benefit [214]. In an affectional RCT with 172 participants undergoing laparoscopic radical prostatectomy randomised to seven days of ciprofloxacin (n=80) or no treatment (n=80) at the time of catheter removal, which occurred at a mean of nine days post-operatively, there was no difference in infective complications recorded at up to four weeks after catheter removal. More isolates obtained from the prophylaxis group (11) were resistant to ciprofloxacin compared to the no treatment group (3) [215]. With regards to catheter insertion, a systematic review and meta-analysis showed that prophylactic antibiotics reduced the rate of bacteriuria and other signs of infection, such as pyuria, fever and gram‐negative isolates in patients’ urine, in surgical patients who undergo bladder drainage for at least 24 hours post-operatively [299].
3.8.4.6. Antibiotic prophylaxis for intermittent self-catheterisation (ISC)
An RCT investigating the effect of antibiotic prophylaxis in patients performing ISC showed that the frequency of symptomatic antibiotic-treated UTI was reduced by 48% using prophylaxis in a cohort of 404 patients performing ISC [300]. However, resistance against the antibiotics used for UTI treatment was more frequent in urinary isolates from the prophylaxis group than in those from the control group at nine to twelve months.
While the literature shows some benefit for reduction of CA-UTI by utilising antibiotics, the routine use of antibiotics for such a common procedure in the healthcare setting would result in an increased usage of antimicrobials. As highlighted in some of the RCTs this strategy is associated with increased antimicrobial resistance. Antibiotic use is the main driving force in the development of antimicrobial resistance. Current antimicrobial stewardship principles would not favour the routine use of antibiotic prophylaxis for either catheter changes or ISC even when UTIs could be prevented [291].
3.8.4.7. Antimicrobial treatment for suspected CAUTI
A urine specimen for culture should be obtained prior to initiating antimicrobial therapy for presumed CA-UTI due to the wide spectrum of potential infecting organisms and the increased likelihood of antimicrobial resistance. The urine culture should be obtained from the freshly placed catheter prior to the initiation of antimicrobial therapy [28]. Based on the global prevalence on infections in urology (GPIU) study, the causative micro-organisms in CA-UTI are comparable with the causative micro-organisms in other systemic UTI; therefore, symptomatic CA-UTIs should be treated according to the recommendations for systemic UTI (see section 3.7.4) [301].
Seven days is the recommended duration of antimicrobial treatment for patients with CA-UTI who have prompt resolution of symptoms, and fourteen days of treatment is recommended for those with a delayed response, regardless of whether the patient remains catheterised or not [28]. A five-day regimen of levofloxacin may be considered in patients with CA-UTI who are not severely ill. Data are insufficient to make such a recommendation about other fluoroquinolones. With the rise in fluoroquinolone resistance, alternative antimicrobial agents should be selected where possible to start empirical therapy based on local microbiological information. A five-day antibiotic regimen with catheter exchange has been shown in one study to be non-inferior to a ten-day regimen with catheter retention on the basis of clinical cure [302].
A three-day antimicrobial regimen may be considered for women aged ≤ 65 years who develop CA-UTI without upper urinary tract symptoms after an indwelling catheter has been removed. If an indwelling catheter has been in place for two weeks at the onset of CA-UTI and is still indicated, the catheter should be replaced to hasten resolution of symptoms and to reduce the risk of subsequent CA-bacteriuria and CA-UTI. If use of the catheter can be discontinued, a culture of a voided mid-stream urine specimen should be obtained prior to the initiation of antimicrobial therapy to help guide treatment [28]. Long-term indwelling catheters should not be changed routinely. Follow appropriate practices for catheter insertion and care [303].
3.8.4.8. Recommendations for disease management and prevention of CA-UTI
| Summary of evidence | LE |
| A systematic review of nineteen different interventions to reduce UTI including catheter discontinuation and limiting catheterisation in nursing home patients reported successful CA-UTI reduction and reduced catheter usage. | 1b |
| A meta-analysis of seven studies investigating RCTs comparing hydrophilic coated to PVC (standard) catheters for IC, found a statistically lower risk ratio (0.84) for the frequency of UTI in the hydrophilic catheter group. | 1a |
| A meta-analysis showed overall benefit for use of prophylaxis for reduction of infective complications after catheter removal; however, results from individual trials were inconsistent with five out of seven trials including the possibility of no benefit. | 1a |
| A subsequent RCT found no benefit of antibiotic prophylaxis for reduction of infective complications at up to four weeks after catheter removal. | 1b |
| Recommendations | Strength rating |
| Treat symptomatic catheter-associated-UTI according to the recommendations for localised and systemic UTI (see section 3.7.4 ). | Strong |
| Take a urine culture prior to initiating antimicrobial therapy in catheterised patients in whom the catheter has been removed. | Strong |
| Do not treat catheter-associated asymptomatic bacteriuria in general. | Strong |
| Treat catheter-associated asymptomatic bacteriuria prior to traumatic urinary tract interventions (e.g. transurethral resection of the prostate). | Strong |
| Replace or remove the indwelling catheter before starting antimicrobial therapy. | Strong |
| Do not apply topical antiseptics or antimicrobials to the catheter, urethra or meatus. | Strong |
| Do not use prophylactic antimicrobials to prevent catheter-associated UTIs. | Strong |
| Do not routinely use antibiotic prophylaxis to prevent clinical UTI after urethral catheter removal. | Weak |
| The duration of catheterisation should be minimal. | Strong |
| Use hydrophilic coated catheters to reduce CA-UTI. | Strong |
| Do not routinely use antibiotic prophylaxis to prevent clinical UTI after urethral catheter removal or in patients performing intermittent self-catheterisation. | Weak |
3.9. Urosepsis
3.9.1. Introduction
Patients with urosepsis should be diagnosed at an early stage. Systemic inflammatory response syndrome (SIRS), characterised by fever or hypothermia, leucocytosis or leukopenia, tachycardia and tachypnoea, has been recognised as a set of alerting symptoms [304,305]; however, SIRS is no longer included in the recent terminology of sepsis (Table 7) [306]. Mortality is considerably increased the more severe the sepsis is.
The treatment of urosepsis involves adequate life-supporting care, appropriate and prompt antimicrobial therapy, adjunctive measures and the optimal management of urinary tract disorders [307]. Source control by decompression of any obstruction and drainage of larger abscesses in the urinary tract is essential [307]. Urologists are recommended to treat patients in collaboration with intensive care and infectious diseases specialists.
Urosepsis is seen in both community-acquired and healthcare associated infections. Nosocomial urosepsis may be reduced by measures used to prevent nosocomial infection, e.g. reduction of hospital stay, early removal of indwelling urinary catheters, avoidance of unnecessary urethral catheterisation, correct use of closed catheter systems, and attention to simple daily aseptic techniques to avoid cross-infection.
Sepsis is diagnosed when clinical evidence of infection is accompanied by signs of systemic inflammation, presence of symptoms of organ dysfunction and persistent hypotension associated with tissue anoxia (Table 7).
3.9.2. Epidemiology, aetiology and pathophysiology
Urinary tract infections can manifest from bacteriuria with limited clinical symptoms to sepsis or severe sepsis, depending on localised and potential systemic extension. It is important to note that a patient can move from an almost harmless state to severe sepsis in a very short time.
Mortality rates associated with sepsis vary depending on the organ source [308] with urinary tract sepsis generally having a lower mortality than that from other sources [309]. Sepsis is more common in men than in women [310]. In recent years, the overall incidence of sepsis arising from all sources has increased by 8.7% per year [308], but the associated mortality has decreased, which suggests improved management of patients (total in-hospital mortality rate fell from 27.8% to 17.9% from 1995 to 2000) [311]. Although the rate of sepsis due to Gram-positive and fungal organisms has increased, Gram-negative bacteria remain predominant in urosepsis [301,312].
In urosepsis, as in other types of sepsis, the severity depends mostly upon the host response.
Patients who are more likely to develop urosepsis include elderly patients, diabetics, immunosuppressed patients, such as transplant recipients and patients receiving cancer chemotherapy or corticosteroids. Urosepsis also depends on local factors, such as urinary tract calculi, obstruction at any level in the urinary tract, congenital uropathy, NLUTD, or endoscopic manoeuvres. However, all patients can be affected by bacterial species that are capable of inducing inflammation within the urinary tract.
3.9.3. Diagnostic evaluation
For diagnosis of systemic symptoms in sepsis either the full Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score, or the quickSOFA score should be applied (Table 7). Microbiology sampling should be applied to urine, two sets of blood cultures [313], and if appropriate drainage fluids. Imaging investigations, such as sonography and CT-scan should be performed early [314].
Table 7: Definition and criteria of sepsis and septic shock [304-306]
| Disorder | Definition |
| Sepsis | Life-threatening organ dysfunction caused by a dysregulated host response to infection. For clinical application, organ dysfunction can be represented by an increase in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score of 2 points or more. For rapid identification a quickSOFA (qSOFA) score was developed: respiratory rate of 22/min or greater, altered mentation, or systolic blood pressure of 100 mmHg or less. |
| Septic shock | Septic shock should be defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by a vasopressor requirement to maintain a mean arterial pressure of 65 mmHg or greater and serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. |
3.9.4. Physiology and biochemical markers
E. coli remains the most prevalent micro-organism. In several countries, bacterial strains can be resistant or multi-resistant and therefore difficult to treat [312]. Most commonly, the condition develops in compromised patients (e.g. those with diabetes or immunosuppression), with typical signs of generalised sepsis associated with local signs of infection.
3.9.4.1. Cytokines as markers of the septic response
Cytokines are involved in the pathogenesis of sepsis [309]. They are molecules that regulate the amplitude and duration of the host inflammatory response. They are released from various cells including monocytes, macrophages and endothelial cells, in response to various infectious stimuli. The complex balance between pro- and anti-inflammatory responses is modified in severe sepsis. An immunosuppressive phase follows the initial pro-inflammatory mechanism. Sepsis may indicate an immune system that is severely compromised and unable to eradicate pathogens or a non-regulated and excessive activation of inflammation, or both. Genetic predisposition is a probable explanation of sepsis in several patients. Mechanisms of organ failure and death in patients with sepsis remain only partially understood [309].
3.9.4.2. Biochemical markers
Procalcitonin is the inactive pro-peptide of calcitonin. Normally, levels are undetectable in healthy humans. During severe generalised infections (bacterial, parasitic and fungal) with systemic manifestations, procalcitonin levels rise [315]. In contrast, during severe viral infections or inflammatory reactions of non-infectious origin, procalcitonin levels show only a moderate or no increase. Mid-regional proadrenomedulline is another sepsis marker. Mid-regional proadrenomedullin has been shown to play a decisive role in the induction of hyperdynamic circulation during the early stages of sepsis and progression to septic shock [316]. Procalcitonin monitoring may be useful in patients likely to develop sepsis and to differentiate from a severe inflammatory status not due to bacterial infection [315,317]. In addition, serum lactate is a marker of organ dysfunction and is associated with mortality in sepsis [318]. Serum lactate should therefore also be monitored in patients with severe infections.
3.9.5. Disease management
3.9.5.1. Prevention
Septic shock is the most frequent cause of death for patients hospitalised for community-acquired and nosocomial infection (20-40%). Urosepsis treatment requires a combination of treatment including source control (obstruction of the urinary tract), adequate life-support care, and appropriate antimicrobial therapy [309,314]. In such a situation, it is recommended that urologists collaborate with intensive care and infectious disease specialists for the best management of the patient.
3.9.5.1.1. Preventive measures of proven or probable efficacy
The most effective methods to prevent nosocomial urosepsis are the same as those used to prevent other nosocomial infections [319,320] they include:
- Isolation of patients with multi-resistant organisms following local and national recommendations.
- Prudent use of antimicrobial agents for prophylaxis and treatment of established infections, to avoid selection of resistant strains. Antibiotic agents should be chosen according to the predominant pathogens at a given site of infection in the hospital environment.
- Reduction in hospital stay. Long inpatient periods before surgery lead to a greater incidence of nosocomial infections.
- Early removal of indwelling urethral catheters, as soon as allowed by the patient’s condition. Nosocomial UTIs are promoted by bladder catheterisation as well as by ureteral stenting [321]. Antibiotic prophylaxis does not prevent stent colonisation, which appears in 100% of patients with a permanent ureteral stent and in 70% of those temporarily stented.
- Use of closed catheter drainage and minimisation of breaks in the integrity of the system, e.g. for urine sampling or bladder wash-out.
- Use of least-invasive methods to release urinary tract obstruction until the patient is stabilised.
- Attention to simple everyday techniques to assure asepsis, including the routine use of protective disposable gloves, frequent hand disinfection, and using infectious disease control measures to prevent cross-infections.
3.9.5.1.2. Appropriate peri-operative antimicrobial prophylaxis
For appropriate peri-operative antimicrobial prophylaxis see section 3.17. The potential side effects of antibiotics must be considered before their administration in a prophylactic regimen.
3.9.5.2. Treatment
Early goal-directed resuscitation was initially shown to improve survival for emergency department patients presenting with septic shock in a randomised, controlled, single-centre study [322]. However, follow-up studies in an improved emergency medicine background have not achieved positive effects with this strategy [323-325]. An individual patient data meta-analysis of the later three multicentre trials concluded that early goal-directed therapy did not result in better outcomes than usual care and was associated with higher hospitalisation costs [326].
3.9.5.2.1. Antimicrobial therapy
Initial empiric antimicrobial therapy should provide broad antimicrobial coverage against all likely causative pathogens and should be adapted on the basis of culture results, once available [307,314]. The dosage of the antimicrobial substances is of paramount importance in patients with sepsis syndrome and should generally be high, with appropriate adjustment for renal function [307]. Antimicrobials must be administered no later than one hour after clinical assumption of sepsis [307].
3.9.5.2.2. Source control
Obstruction in the urinary tract is the most frequent urological source of urosepsis. Drainage of obstruction and abscesses, and removal of foreign bodies, such as urinary catheters or stones is therefore the most important source control strategy. These are key components of the strategy. This condition is an absolute emergency.
3.9.5.2.3. Adjunctive measures
The most important adjunctive measures in the management of sepsis are the following [307,314]:
- fluid therapy with crystalloids, or albumin, if crystalloids are not adequately increasing blood pressure: passive leg raising-induced changes in cardiac output and in arterial pulse pressure are predictors of fluid responsiveness in adults [327];
- as vasopressors norepinephrine should be used primarily, dobutamine in myocardial dysfunction;
- hydrocortisone should be given only if fluid and vasopressors do not achieve a mean arterial pressure of ≥ 65 mmHg;
- blood products should be given to target a haemoglobin level of 7-9 g/dL;
- mechanical ventilation should be applied with a tidal volume 6 mL/kg and plateau pressure ≤ 30 cm H2O and a high positive end-expiratory pressure;
- sedation should be given minimally, neuromuscular blocking agents should be avoided;
- glucose levels should be target at ≤ 180 mg/dL;
- deep vein thrombosis prevention should be given with low-molecular weight heparin subcutaneously;
- stress ulcer prophylaxis should be applied in patients at risk, using proton pump inhibitors;
- enteral nutrition should be started early (< 48 hours).
In conclusion, sepsis in urology remains a severe situation with a considerable mortality rate. A recent campaign, ‘Surviving Sepsis Guidelines’, aims to reduce mortality by 25% in the next years [307,314,328]. Early recognition of the symptoms may decrease the mortality by timely treatment of urinary tract disorders, e.g. obstruction, or urolithiasis. Adequate life-support measures and appropriate antimicrobial treatment provide the best conditions for improving patient survival. The prevention of sepsis is dependent on good practice to avoid nosocomial infections and using antimicrobial prophylaxis and therapy in a prudent and well-accepted manner.
3.9.5.3. Summary of evidence and recommendations for the diagnosis and treatment of urosepsis
| Summary of evidence | LE |
| Initial high dose empiric antimicrobial therapy, administered within the first hour, should provide broad antimicrobial coverage against all likely causative pathogens and should be adapted on the basis of culture results, once available. | 2b |
| Source control interventions should be implemented as soon as possible to control or eliminate diagnosed and/or suspected infectious foci. | 3 |
| Recommendations | Strength rating |
| Perform the quickSOFA score to identify patients with potential sepsis. | Strong |
| Take a urine culture and two sets of blood cultures before starting antimicrobial treatment. | Strong |
| Administer parenteral high dose broad spectrum antimicrobials within the first hour after clinical assumption of sepsis. | Strong |
| Adapt initial empiric antimicrobial therapy on the basis of culture results. | Strong |
| Initiate source control including removal of foreign bodies, decompression of obstruction and drainage of abscesses in the urinary tract. | Strong |
| Provide immediate adequate life-support measures. | Strong |
Table 8: Suggested regimens for antimicrobial therapy for urosepsis.
| Antimicrobials | Daily dose | Duration of therapy |
| Cefotaxime | 2 g t.i.d | 7-10 days Longer courses are appropriate in patients who have a slow clinical response |
| Ceftazidime | 1-2 g t.i.d | |
| Ceftriaxone | Standard dosage: 2 g q.d High dosage: 2 g iv b.i.d | |
| Cefepime | Standard dosage: 1 g iv t.i.d or 2 g iv bid High dosage: 2 g iv t.i.d | |
| Piperacillin/tazobactam | Standard dosage: 4 g piperacillin + 0.5 g tazobactam x 4 iv 30-minute infusion or x 3 iv by extended 4-hour infusion High dosage: 4 g piperacillin + 0.5 g tazobactam x 4 iv by extended 3-hour infusion | |
| Ceftolozane/tazobactam | 1.5 g t.i.d | |
| Ceftazidime/avibactam | 2.5 g t.i.d | |
| Gentamicin* | 6-7 mg/kg q.d | |
| Amikacin* | 25 - 30 mg/kg q.d | |
| Ertapenem | 1 g q.d | |
| Imipenem/cilastatin | 0.5 g q.i.d | |
| Meropenem | Standard dosage: 1 g t.i.d High dosage: 2 g iv t.i.d |
* Not studied as monotherapy in urosepsis
b.i.d = twice daily; t.i.d = three times daily; q.d = every day; iv = intravenously.
3.10. Urethritis
3.10.1. Introduction
Urethritis can be of either infectious or non-infectious origin. Inflammation of the urethra presents usually with subjective symptoms (dysuria, alguria, burning, itching and pain around the distal urethra and external urethral meatus) and clinical signs (urethral discharge, erythema around the external urethral meatus and inguinal lymphadenopathy). These symptoms and signs must be distinguished from other infections of the lower urinary tract. Urethral infection is typically spread by sexual contact. The following recommendations are based on the consultation draft of the S3 guideline on urethritis “Management of Urethritis in Male Adolescents and Adults” (AWMF registry number 013-099) [329].
3.10.2. Epidemiology, aetiology and pathogenesis
In most cases, urethritis is caused by sexually transmitted bacterial infections, the most common of which are C. trachomatis, N. gonorrhoeae and M. genitalium. It should be noted that pathogens whose relevance as a cause of urethritis symptoms is questionable or must be determined on a case-by-case basis, such as U. urealyticum, U. parvum and M. hominis, are also frequently detected. Urethral infections with more than one pathogen are common. Empirical antibiotic treatment must therefore take into account the potential effects of targeting the primary pathogen on any coexisting infections. From a therapeutic and clinical point of view, gonorrhoeal urethritis (GU) caused by Neisseria gonorrhoeae must be differentiated from non-gonococcal urethritis (NGU). Non-gonococcal urethritis is a non-specific diagnosis that can have many infectious aetiologies. Causative pathogens include Chlamydia trachomatis, Mycoplasma genitalium, Ureaplasma urealyticum and Trichomonas vaginalis. The role of Ureaplasma spp. as urethritis causative pathogens is controversial. Recent data suggests that U. urealyticum, but not U. parvum is an aetiological agent in NGU [330]. The prevalence of isolated causative pathogens are: C. trachomatis 11-50%; M. genitalium 6-50%; Ureaplasmas 5-26%; T. vaginalis 1-20%; and adenoviruses 2-4% [331].
The causative microorganisms either remain extracellularly on the epithelial layer or penetrate into the epithelium (N. gonorrhoeae and C. trachomatis) and cause pyogenic infection. Although arising from urethritis, chlamydiae and gonococci can spread further through the urogenital tract to cause epididymitis in men or cervicitis, endometritis and salpingitis in women [332].
3.10.3. Diagnostic evaluation
In symptomatic patients, the diagnosis of urethritis can be made on the basis of the presence of any of the following criteria [331,332]:
- Mucoid (watery-clear), mucopurulent (whitish-opaque urethral discharge), or purulent (yellow-green) urethral discharge.
- Gram or methylene-blue staining of urethral discharge showing inflammation. Five or more polymorphonuclear leucocytes (PMNL) per high power field (HPF) is the historical cut-off for the diagnosis of urethritis. A cut-off of ≥ 2 PMNL/HPF has recently been proposed recently based on improved diagnostic accuracy [333-336], but this was not supported by other studies [337]. Therefore, in line with the 2016 European Guideline on the management of NGU [331] the use of ≥ 5 PMNL/HPF cut-off is recommended until the benefit of alternative cut-off levels is confirmed.
- The presence of ≥ 10 PMNL/HPF in the sediment from a spun first-void urine sample or a positive leukocyte esterase test in first-void urine.
Evidence of urethral inflammation on the Gram stain of urethral secretions with intracellular gonococci as Gram-negative diplococci indicates GU. Non-gonococcal urethritis is confirmed when staining of urethral secretions indicates inflammation in the absence of intracellular diplococci. Clinicians should always perform point-of-care diagnostics (e.g. Gram staining, first-void urine with microscopy, leukocyte esterase test) when available to obtain objective evidence of urethral inflammation and to guide treatment [331,332,338]. Recent studies have shown that processing time of point-of-care diagnostics is highly relevant in terms of patient compliance and real-life applicability [339,340].
3.10.4. Urethral swab, urinalysis, NAAT
In cases of urethral discharge and suspected GU, a meatal/urethral swab should be taken for microbiological culture and N. gonorrhoeae resistance testing. Regardless of the initial classification of urethritis as GU or NGU, a nucleic acid amplification test (NAAT) should be performed to detect N. gonorrhoeae, M. genitalium and C. trachomatis [331,341]. For molecular genetic diagnosis of N. gonorrhoeae, C. trachomatis and M. genitalium in cases of suspected urethral infection, both meatal/urethral swabs and first-void urine can be used. The sensitivity and specificity of NAAT are better than the other tests available for the diagnosis of chlamydial and gonococcal infections [342,343]. The performance of first-catch urine is non-inferior to urethral swabs [342]. In case of delayed treatment, if a NAAT is positive for gonorrhoea, a urethral swab culture should be performed prior to treatment to assess the antimicrobial resistance profile of the infecting strain [332]. N. gonorrhoeae and C. trachomatis cultures are mainly used to assess treatment failures and to monitor the development of resistance to current treatment. Trichomonas spp. can usually be identified microscopically [332] or by NAATs [344].
Non-gonococcal urethritis is considered persistent if symptoms do not resolve within three to four weeks following treatment. In this case, NAAT for urethritis pathogens including T. vaginalis should be performed four weeks after completion of therapy [331,345].
3.10.5. Disease management
In the context of increasing antimicrobial resistance among potentially relevant pathogens, the question is increasingly raised whether empirical antibiotic therapy for urethritis, initiated before pathogen identification, remains appropriate. However, current international guidelines and reviews on urethritis in men generally recommend immediate empirical antibiotic therapy, based on the distinction between GU and NGU. All sexual partners at risk should be assessed and treated whilst maintaining patient confidentiality [331,346].
3.10.5.1. Suspected gonococcal urethritis
IF GU is suspected, a combination treatment using two antimicrobials is recommended [332]. Ceftriaxone 1-2 g intramuscularly or intravenously with doxycycline 100 mg b.i.d, p.o., seven days should be used as first-line treatment. In cases of suspected GU with contraindications to doxycycline, and if follow-up cannot be ensured, an alternative approach is a combined empirical treatment with ceftriaxone as outlined above and azithromycin administered orally according to a four-day regimen (day 1: 1 g; days 2–4: 500 mg orally).
3.10.5.2. Suspected non-gonococcal urethritis
For NGU without an identified pathogen, oral doxycycline 100 mg twice daily for seven days should be used as first-line treatment. In cases of suspected NGU with contraindications to doxycycline, and if follow-up cannot be ensured, an alternative empirical treatment with oral azithromycin following the four-day regimen (day 1: 1 g; days 2–4: 500 mg orally) should be considered.
3.10.5.3. Gonococcal urethritis
In cases where N. gonorrhoeae is detected, first-line therapy should consist of a single dose of ceftriaxone 1-2 g given intravenously or intramuscularly. In cases of contraindications to cephalosporins and detection of N. gonorrhoeae with culture-confirmed sensitivity to azithromycin, treatment with oral azithromycin should be administered as follows: If co-infection with M. genitalium has been excluded: a single dose of 1 g, if co-infection with M. genitalium cannot be excluded: the 4-day regimen (day 1: 1 g; days 2–4: 500 mg daily). In cases of contraindications to cephalosporins and detection of N. gonorrhoeae with molecularly or culture-confirmed sensitivity to ciprofloxacin, a single oral dose of ciprofloxacin 500 mg may be considered. Other alternative regimens for the treatment of GU have been studied and are shown in Table 10 [332,347-354].
3.10.5.4. Non-gonococcal urethritis
3.10.5.4.1. Chlamydia trachomatis
When C. trachomatis is detected, first-line therapy should consist of doxycycline 100 mg, administered orally twice daily for seven days. If C. trachomatis is detected and doxycycline is contraindicated, treatment with oral azithromycin should be considered. If co-infection with M. genitalium has been excluded, a single dose of 1 g is recommended, if co-infection with M. genitalium cannot be ruled out, azithromycin should be administered according to the four-day regimen, with 1 g on the first day followed by 500 mg daily on days 2 through 4. Fluoroquinolones, such as ofloxacin or levofloxacin, may be used as second-line treatment only in selected cases where the other agents cannot be used [355].
3.10.5.4.2. Mycoplasma genitalium
In cases of M. genitalium detection without molecular resistance testing or without the detection of macrolide resistance-associated mutations (MRAM), first-line therapy should consist of azithromycin administered according to the four-day regimen: 1 g on day 1, followed by 500 mg daily on days 2 through 4. In cases of M. genitalium detection, treatment with moxifloxacin 400 mg, taken orally once daily for seven days, is recommended if contraindications to azithromycin are present, if molecular genetic testing confirms the presence of macrolide resistance-associated mutations (MRAM), if the transmission setting suggests a high likelihood of azithromycin resistance, or if there was no response to prior treatment with azithromycin [331,332,356]. If there is a failure to or contraindications to azithromycin and moxifloxacin, treatment with sitafloxacin 100 mg, taken orally twice daily for seven days, may be considered in cases of M. genitalium is detected.
3.10.5.4.3. Mycoplasma hominis and Ureaplasma spp.
In cases of urethral detection of U. urealyticum, it is often a colonisation without clinical relevance. The indication for treatment should be evaluated on an individual basis in the absence of other pathogens. Urethral detection of M. hominis and U. parvum generally indicates colonisation without clinical relevance and does not warrant treatment. If U. urealyticum is identified as the sole causative agent of symptomatic urethritis, treatment with doxycycline 100 mg, administered orally twice daily for seven days, is recommended. If U. urealyticum is identified as the sole cause of symptomatic urethritis and there are contraindications to doxycycline, treatment with a single oral dose of azithromycin 1 g is recommended [331,357].
3.10.5.4.4. Trichomonas vaginalis
If T. vaginalis is detected, first-line therapy should consist of a single oral dose of metronidazole 1.5 g–2 g. In cases where contraindications to metronidazole exist, an alternative treatment with tinidazole 2,000 mg orally may be considered. For treatment options for persistent or recurrent T. vaginalis infection, refer to the review of Sena et al. [344].
3.10.6. Follow-up
Individuals with a clinical diagnosis of acute urethritis should be advised to abstain from sexual activity for at least one week after completion of antibiotic therapy, or longer if symptoms persist. Sexual partners from the weeks before the onset of symptoms should be informed of the diagnosis and the need for appropriate testing and, if necessary, treatment. If symptoms persist for more than two weeks after completion of antibiotic therapy, or if symptoms recur, follow-up medical consultation is recommended. For the initial diagnosis of N. gonorrhoeae, C. trachomatis or M. genitalium, a follow-up cure test should be scheduled six to twelve weeks after completion of antibiotic therapy. In addition, when urethritis is diagnosed, testing for HIV and other sexually transmitted infections (STIs) should be offered in addition to the urethritis diagnostic procedures recommended in this guideline [358].
3.10.7. Summary of evidence and recommendations for the diagnostic evaluation and antimicrobial treatment of urethritis
| Summary of evidence | LE |
| A Gram stain of urethral discharge or a urethral smear that shows ≥ 5 leukocytes per high power field (× 1,000) and gonococci located intracellularly as Gram-negative diplococci, indicates gonococcal urethritis. | 3b |
| Validated NAATs of first-void urine samples have better sensitivity and specificity than any of the other tests available for the diagnosis of chlamydial and gonococcal infections. | 2a |
| For GU, dual treatment with ceftriaxone and azithromycin is the most effective combination. | 2a |
| In case of urogenital C. trachomatis infection in men, azithromycin is probably less effective than doxycycline for microbiological failure. | 1a |
| In case of U. urealyticum infection the efficacy of doxycycline 100 mg twice for seven days is similar to azithromycin 1 g single dose treatment. | 2a |
| Recommendations | Strength rating |
| Perform a Gram stain of urethral discharge or a urethral smear to preliminarily diagnose gonococcal urethritis. | Strong |
| Perform a validated nucleic acid amplification test (NAAT) on a first-void urine sample or urethral smear prior to empirical treatment to diagnose chlamydial and gonococcal infections. | Strong |
| If possible, delay treatment until the results of the NAATs are available to guide treatment choice in patients with mild symptoms. | Strong |
| Perform a urethral swab culture, prior to initiation of treatment, in patients with a positive NAAT for gonorrhoea to assess the antimicrobial resistance profile of the infective strain. | Strong |
| Use a pathogen directed treatment based on local resistance data. | Strong |
| Sexual partners should be treated, while maintaining patient confidentiality. | Strong |
Table 9: Suggested regimens for antimicrobial therapy for urethritis
| Suspected | Antimicrobial | Dosage & Duration of therapy | Alternative regimens |
| Gonococcal infection | Ceftriaxone Doxycycline | 1-2 g i.m. or i.v.*, SD 100 mg b.i.d, p.o., 7 days | In case of doxycycline allergy, in combination with ceftriaxone: Azithromycin 4-day regimen: Day 1: 1 g; |
| Non-Gonococcal infection | Doxycycline | 100 mg b.i.d, p.o., 7 days | Azithromycin 4-day regimen: Day 1: 1 g; Days 2–4: 500 mg p.o |
Table 10: Regimens for antimicrobial therapy for urethritis with causing pathogen detected
| Pathogen | Antimicrobial | Dosage & Duration of therapy | Alternative regimens |
| Neisseria gonorrhoeae | Ceftriaxone Doxycycline | 1-2 g i.m. or i.v.*, SD 100 mg b.i.d, p.o., 7 days |
In case of doxycycline allergy, in combination with ceftriaxone: Azithromycin 4-day regimen: Day 1: 1 g; Days 2–4: 500 mg orally |
| Chlamydia trachomatis | Doxycycline | 100 mg b.i.d, p.o., for 7 days |
|
| Mycoplasma genitalium | Azithromycin | 4-day regimen: Day 1: 1 g; Days 2–4: 500 mg p.o. | In case of macrolide resistance:
|
| Ureaplasma urealyticum | Doxycycline | 100 mg b.i.d, p.o., 7 days | Azithromycin 1 g p.o., SD |
| Trichomonas vaginalis | Metronidazole | 1.5-2 g p.o., SD | Tinidazole 2 g p.o., SD |
SD = single dose; b.i.d = twice daily; q.d = everyday; p.o. = orally; i.m. = intramuscular; i.v. intravenously.
* Despite the lack of RCTs there is increasing evidence that Intravenous treatment with ceftriaxone is safe and effective for the treatment of gonorrhoeal infections and avoids the discomfort of an intramuscular injection for patients [359]
3.11. Bacterial Prostatitis
3.11.1. Introduction
Bacterial prostatitis is a clinical condition caused by bacterial pathogens. It is recommended that urologists use the classification suggested by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH), in which bacterial prostatitis, with confirmed or suspected infection, is distinguished from chronic pelvic pain syndrome (CPPS) (Table 11) [360-362].
Table 11: Classification of prostatitis and CPPS according to NIDDK/NIH [360-362]
| Type | Name and description |
| I | Acute bacterial prostatitis (ABP) |
| II | Chronic bacterial prostatitis (CBP) |
| III | Chronic non-bacterial prostatitis – CPPS |
| IIIA | Inflammatory CPPS (white cells in semen/EPS/VB3) |
| IIIB | Non-inflammatory CPPS (no white cells in semen/EPS/VB3) |
| IV | Asymptomatic inflammatory prostatitis (histological prostatitis) |
CPPS = chronic pelvic pain syndrome; EPS = expressed prostatic secretion; VB3 = voided bladder urinespecimen 3 (urine following prostatic massage).
3.11.2. Evidence Summary
A systematic literature search from 1980 until June 2017 was performed. One systematic review [363], six RCTs [364-369], two narrative reviews [370,371], one prospective cohort study [372], two prospective cross-sectional studies [373,374], and one retrospective cohort study [366], were selected from 856 references.
A retrospective study [375], investigated the potential role of unusual pathogens in prostatitis syndrome in 1,442 patients over a four-year period. An infectious aetiology was determined in 74.2% of patients; C. trachomatis, T. vaginalis and U. urealyticum infections were found in 37.2%, 10.5% and 5% of patients, respectively, whilst E. coli infection was found in only 6.6% of cases. Cross sectional studies confirmed the validity of the Meares and Stamey test to determine the bacterial strain and targeted antibiotic therapies [373,374]. The evidence levels were good, in particular those regarding information on atypical strains, epidemiology and antibiotic treatments.
A systematic review on antimicrobial therapy for CBP [363] compared multiple antibiotic regimens from eighteen selected studies enrolling a total of 2,196 patients. The role of fluoroquinolones as first line agents was confirmed with no significant differences between levofloxacin, ciprofloxacin and prulifloxacin in terms of microbiological eradication, clinical efficacy and adverse events. The efficacy of macrolides and tetracyclines on atypical pathogens was confirmed.
Randomised controlled trials on combined treatments [368,369] indicated that the combination of plants/herbal extracts or PDE5Is with antibiotics may improve quality of life and symptoms in patients with CBP; however, the number of enrolled patients was inadequate to obtain definitive conclusions.
A review of treatment of bacterial prostatitis [370] indicated that the treatment of CBP is hampered by the lack of an active antibiotic transport mechanism into infected prostate tissue and fluids. The review underlined the potential effect of different compounds in the treatment of ABP and CBP on the basis of over 40 studies on the topic.
One RCT compared the effects of two different metronidazole regimens for the treatment of CBP caused by T. vaginalis [367]. Metronidazole 500 mg three times daily for fourteen days was found to be efficient for micro-organism eradication in 93.3% of patients with clinical failure in 3.33% of cases. The evidence question addressed was: In men with NIDDK/NIH Category I or II prostatitis, what is the best antimicrobial treatment strategy for clinical resolution and eradication of the causative pathogen?
3.11.3. Epidemiology, aetiology and pathogenesis
Prostatitis is a common diagnosis, but less than 10% of cases have proven bacterial infection [228]. Enterobacterales, especially E. coli, are the predominant pathogens in ABP [376]. In CBP, the spectrum of species is wider and may include atypical micro-organisms [370]. In patients with immune deficiency or HIV infection, prostatitis may be caused by fastidious pathogens, such as M. tuberculosis, Candida spp. and other rare pathogens, such as Coccidioides immitis, Blastomyces dermatitidis, and Histoplasma capsulatum [377]. The significance of identified intracellular bacteria, such as C. trachomatis, is uncertain [378]; however, two studies have highlighted its possible role as a causative pathogen in CBP [379,380].
3.11.4. Diagnostic evaluation
3.11.4.1. History and symptoms
Acute bacterial prostatitis usually presents abruptly with voiding symptoms and distressing but poorly localised pain. It is often associated with malaise and fever. Transrectal prostate biopsy increases the risk of ABP despite antibiotic prophylaxis and antiseptic prevention procedures [364]. Chronic bacterial prostatitis is defined by symptoms that persist for at least three months [381-383]. The predominant symptoms are pain at various locations including the perineum, scrotum, penis and inner part of the leg as well as LUTS [360-362].
3.11.4.2. Symptom questionnaires
In CBP, symptoms appear to have a strong basis for use as a classification parameter [384]. Prostatitis symptom questionnaires have therefore been developed to assess severity and response to therapy [384,385]. They include the validated Chronic Prostatitis Symptom Index (CPSI); however, its usefulness in clinical practice is uncertain [372].
3.11.4.3. Clinical findings
In ABP, the prostate may be swollen and tender on DRE. Prostatic massage should be avoided as it can induce bacteraemia and sepsis. Urine dipstick testing for nitrite and leukocytes has a positive predictive value of 95% and a negative predictive value of 70% [386]. Blood culture and complete blood count are useful in ABP. Imaging studies can detect a suspected prostatic abscess [370].
In case of longer lasting symptoms, CPPS as well as other urogenital and anorectal disorders must be taken into consideration. Symptoms of CBP or CPPS can mask prostate tuberculosis. Pyospermia and hematospermia in men in endemic regions or with a history of tuberculosis should trigger investigation for urogenital tuberculosis.
3.11.4.4. Urine cultures and expressed prostatic secretion
The most important investigation in the evaluation of a patient with ABP is mid-stream urine culture [370]. In CBP, quantitative bacteriological localisation cultures and microscopy of the segmented urine and expressed prostatic secretion (EPS), as described by Meares and Stamey [387], are still important investigations to categorise clinical prostatitis [373,374]. Accurate microbiological analysis of samples from the Meares and Stamey test may also provide useful information on the presence of atypical pathogens such as C. trachomatis, T. vaginalis and U. urealiticum [375]. The two-glass test has been shown to offer similar diagnostic sensitivity to the four-glass test [388].
3.11.4.5. Prostate biopsy
Prostate biopsies cannot be recommended as routine work-up and are not advisable in patients with untreated bacterial prostatitis due to the increased risk of sepsis.
3.11.4.6. Other tests
Transrectal US may reveal endoprostatic abscesses, calcification in the prostate, and dilatation of the seminal vesicles; however, it is unreliable as a diagnostic tool for prostatitis [389].
3.11.4.7. Additional investigations
3.11.4.7.1. Ejaculate analysis
Performing an ejaculated semen culture improves the diagnostic utility of the four-glass test [373]; however, semen cultures are more often positive than EPS cultures in men with non-bacterial prostatitis [374]. Bladder outflow and urethral obstruction should always be considered and ruled out by uroflowmetry, retrograde urethrography, or endoscopy.
3.11.4.7.2. First-void urine sample
First-void urine is the preferred specimen for the diagnosis of urogenital C. trachomatis infection in men by NAATs, since it is non-invasive and yet allows the detection of infected epithelial cells and associated C. trachomatis particles [390].
3.11.4.7.3. Prostate specific antigen (PSA)
Prostate specific antigen is increased in about 60% and 20% of men with ABP and CBP, respectively [371]. The PSA level decreases after antibiotic therapy (which occurs in approximately 40% of patients) and correlates with clinical and microbiological improvement [365]. Measurement of free and total PSA adds no practical diagnostic information in prostatitis [391].
3.11.4.8. Summary of evidence and recommendations for the diagnosis of bacterial prostatitis
| Summary of evidence | LE |
| Urine dipstick testing for nitrite and leukocytes has a positive predictive value of 95% and a negative predictive value of 70% in patients with ABP. | 3 |
| The four-glass Meares and Stamey test is the optimum test for diagnosis of CBP. The two-glass test has been shown to offer similar diagnostic sensitivity in a comparison study. | 2b |
| First-void urine is the preferred specimen for the diagnosis of urogenital C. trachomatis infection in men by NAATs. | 2b |
| Transrectal ultrasound is unreliable and cannot be used as a diagnostic tool in prostatitis. | 3 |
| Semen culture sensitivity is reported to be approximately 50%; therefore, it is not routinely part of the diagnostic assessment of CBP. | 3 |
| Prostate specific antigen levels may be elevated during active prostatitis; therefore, PSA testing should be avoided as it offers no practical diagnostic information for prostatitis. | 3 |
| Recommendations | Strength rating |
| Do not perform prostatic massage in acute bacterial prostatitis (ABP). | Strong |
| Take a mid-stream urine dipstick to check nitrite and leukocytes in patients with clinical suspicion of ABP. | Weak |
| Take a mid-stream urine culture in patients with ABP symptoms to guide diagnosis and tailor antibiotic treatment. | Weak |
| Take a blood culture and a total blood count in patients presenting with ABP. | Weak |
| Perform accurate microbiological evaluation for atypical pathogens such as Chlamydia trachomatis or Mycoplasmata in patients with chronic bacterial prostatitis (CBP). | Weak |
| Perform the Meares and Stamey 2- or 4-glass test in patients with CBP. | Strong |
| Perform transrectal ultrasound in selected cases to rule out the presence of prostatic abscess. | Weak |
| Do not routinely perform microbiological analysis of the ejaculate alone to diagnose CBP. | Weak |
3.11.5. Disease management
3.11.5.1. Antimicrobials
Antimicrobials are life-saving in ABP and recommended in CBP. Culture-guided antibiotic treatments are the optimum standard; however, empirical therapies should be considered in all patients with ABP.
In ABP, parenteral administration of high doses of bactericidal antimicrobials, such as broad-spectrum penicillins, a third-generation cephalosporin or fluoroquinolones, is recommended [392]. For initial therapy, any of these antimicrobials may be combined with an aminoglycoside [376-385,392-396]. Ancillary measures include adequate fluid intake and urine drainage [228]. After normalisation of infection parameters, oral therapy can be substituted and continued for a total of two to four weeks [397].
Fluoroquinolones, despite the high resistance rates of uropathogens, are recommended as first-line agents in the empirical treatment of CBP because of their favourable pharmacokinetic properties [398], their generally good safety profile and antibacterial activity against Gram-negative pathogens including P. aeruginosa and C. trachomatis [363,399]. However, increasing bacterial resistance is a concern. Azithromycin and doxycycline are active against atypical pathogens such as C. trachomatis and genital mycoplasmata [366,375]. Levofloxacin did not demonstrate significant clearance of C. trachomatis in patients with CBP [400]. Metronidazole treatment is indicated in patients with T. vaginalis infections [367].
Duration of fluoroquinolone treatment must be at least fourteen days while azithromycin and doxycycline treatments should be extended to at least three to four weeks [366,375]. In CBP, antimicrobials should be given for four to six weeks after initial diagnosis [370]. If intracellular bacteria have been detected, macrolides or tetracyclines should be given [363,398,401].
3.11.5.2. Intraprostatic injection of antimicrobials
This treatment has not been evaluated in controlled trials and should not be considered [402,403].
3.11.5.3. Combined treatments
A combination of fluoroquinolones with various herbal extracts may attenuate clinical symptoms without increasing the rate of adverse events [368]. However, a combination of fluoroquinolones with vardenafil did not improve microbiological eradication rates or attenuated pain or voiding symptoms in comparison with fluoroquinolone treatment alone [369].
3.11.5.4. Drainage and surgery
Approximately 10% of men with ABP will experience urinary retention [404] which can be managed by urethral or suprapubic catheterisation. However, recent evidence suggests that suprapubic catheterisation can reduce the risk of development of CBP [405].
In case of prostatic abscess, both drainage and conservative treatment strategies appear feasible [406]; however, the abscess size may matter. In one study, conservative treatment was successful if the abscess cavities were < 1 cm in diameter, while larger abscesses were better treated by single aspiration or continuous drainage [407].
3.11.5.5. Summary of evidence and recommendations for the disease management of bacterial prostatitis
| Summary of evidence | LE |
| The treatment regimen for ABP is based on clinical experience and a number of uncontrolled clinical studies. For systemically ill patients with ABP, parenteral antibiotic therapy is preferable. After normalisation of infection parameters, oral therapy can be substituted and continued for a total of two to four weeks. | 3 |
| The role of fluoroquinolones as first-line agents for antimicrobial therapy for CBP was confirmed in a systematic review, with no significant differences between levofloxacin, ciprofloxacin and prulifloxacin in terms of microbiological eradication, clinical efficacy and adverse events. | 1a |
| Metronidazole 500 mg three times daily for fourteen days was found to be efficient for eradication in 93.3% of patients with T. vaginalis CBP. | 1b |
| In patients with CBP caused by obligate intracellular pathogens, macrolides showed higher microbiological and clinical cure rates compared to fluoroquinolones. | 1a |
| Clinicians should consider local drug-resistance patterns when choosing antibiotics. | 3 |
| Recommendations | Strength rating |
| Acute bacterial prostatitis | |
| Treat acute bacterial prostatitis according to the recommendations for systemic UTIs (see section 3.7.4). | Strong |
| Chronic bacterial prostatitis (CBP) | |
| Prescribe a fluoroquinolone (e.g. ciprofloxacin, levofloxacin) as first-line treatment for CBP. | Strong |
| Prescribe a macrolide (e.g. azithromycin) or a tetracycline (e.g. doxycycline) if intra-cellular bacteria have been identified as the causative agent of CBP. | Strong |
| Prescribe metronidazole in patients with Trichomonas vaginalis CBP. | Strong |
Table 12: Suggested regimens for antimicrobial therapy for chronic bacterial prostatitis
| Antimicrobial | Daily dose | Duration of therapy | Comments |
| Fluoroquinolone | Optimal oral daily dose | 4-6 weeks | |
| Doxycycline | 100 mg b.i.d | 10 days | Only for C. trachomatis or mycoplasma infections |
| Azithromycin | 500 mg once daily | up to 3 weeks | Only for C. trachomatis infections |
| Metronidazole | 500 mg t.i.d | 14 days | Only for T. vaginalis infections |
b.i.d = twice daily; t.i.d = three times daily.
3.11.6. Follow-up
In asymptomatic post-treatment patients, routine urinalysis and/or urine culture is not mandatory as there are no validated tests of cure for bacterial prostatitis except for cessation of symptoms [370]. In patients with persistent symptoms and repeated positive microbiological results for sexually transmitted infectious pathogens, microbiological screening of the patient’s partner/s is recommended. Antibiotic treatments may be repeated with a more prolonged course, higher dosage and/or different compounds [370].
3.12. Acute Epididymitis
3.12.1. Epidemiology, Aetiology and Pathophysiology
Epididymitis is a common condition with incidence ranging from 25 to 65 cases per 10,000 adult males per year and can be acute, chronic or recurrent [408]. Acute epididymitis is clinically characterised by unilateral pain, palpable swelling and increased temperature of the epididymis, which worsen over several days. The testis and scrotal skin may be involved. The pain is typically localised to the posterior aspect of the testis affected. Acute epididymitis is generally caused by STIs (sexually transmitted epididymitis) or Enterobacterales. Pathogens migrate from the urethra or bladder and can be identified by appropriate diagnostics in up to 90% of patients [409].
The predominant pathogens isolated in sexually transmitted epididymitis in otherwise healthy young and sexually active biological men are Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma genitalium. Acute epididymitis caused by an STI is usually accompanied by urethritis and discharge, which in case of gonorrhea is thick, green, white or yellow and very productive. Non-sexually transmitted epididymitis is typically caused by Enterobacterales (e.g. E. coli) [410]. Older patients with a history of urinary tract infection are most commonly affected. Risk factors include benign prostatic hyperplasia, neurogenic bladder or recent instrumentation. Men who have unprotected anal intercourse are at higher risk of epididymitis caused by Enterobacterales [411]. The mumps virus should be considered if there are bilateral scrotal involvement, viral prodromal symptoms and salivary gland enlargement. Tuberculous epididymitis may occur, typically as chronic epididymitis, in high-risk groups such as men with immunodeficiency and those from high prevalence countries, it frequently results in a discharging scrotal sinus. Brucella or Candida spp. are rare possible pathogens. Torsion of the spermatic cord (testicular torsion) is the most important differential diagnosis in boys and young men.
3.12.2. Diagnostic Evaluation
Culture of a mid-stream specimen of urine should be performed and any previous urine culture results should be checked. Sexually transmitted infections including C. trachomatis or N. gonorrhoeae should be detected by NAAT on first voided urine or urethral swab. A urethral swab or smear might be performed for Gram staining and culture of N. gonorrhoeae, when available [408,412,413]. Detection of these pathogens should be reported according to local procedures. All patients with probable STIs should be screened for other STIs. Patients with Enterobacterales may require investigation for lower urinary tract abnormalities. If tuberculous epididymitis is suspected, three sequential early morning urine samples should be cultured for acid-fast bacilli (AFB) and sent for screening by NAAT for M. tuberculosis DNA [414]. If appropriate prostate secretion, ejaculate, discharge from a draining scrotal fistula, as well as fine needle aspiration and biopsy specimens, should be investigated using microscopy, AFB culture and NAAT. Scrotal ultrasound is more accurate for the diagnose of acute epididymitis than urinalysis alone [415] and may also be beneficial for the exclusion of other pathologies [416].
3.12.3. Disease Management
Empirical antimicrobial therapy has to be chosen with consideration of the most probable pathogen and degree of penetration into the inflamed epididymis and may need to be varied according to local pathogen sensitivities and guidance. Generally, both C. trachomatis and Enterobacterales should be covered initially and the regimen modified according to pathogen identification. Doxycycline and fluoroquinolones have good clinical and microbiological cure rates in patients with suspected C. trachomatis or M. genitalium and both achieve adequate levels in inflamed male genital tissues with oral dosing. Macrolide antibiotics such as azithromycin are effective against C. trachomatis but have not been tested in epididymitis; however, initial pharmacokinetic studies suggest that azithromycin may effectively penetrate epididymal tissue when given in multiple doses [417]. Fluoroquinolones remain effective for oral treatment of Enterobacterales although resistance is increasing and local advice should be sought. Fluoroquinolones should not be considered for gonorrhoea. Single parenteral dose of ceftriaxone is effective against N. gonorrhoeae. However, current resistance patterns and local public health recommendations should guide choice of agent. Patients with likely or proven STI should be assessed at fourteen days to check cure and ensure tracing and treatment of contacts according to local public health recommendations. Nonsteroidal anti-inflammatory drugs and local cooling can be used to manage pain and swelling. Elevating the scrotum may also help reduce discomfort. Men with suspected STI should be informed of the risks to others and advised not to have sex until free of infection. In exceptional cases, individuals with epididymitis may experience severe pain, fever, a testicular abscess, or sign of systemic illness. These patients should be admitted to the hospital for intravenous antibiotic treatment and fluid replacement.
3.12.4. Evidence Summary
Relating to this chapter, four guidelines based on systematic reviews were identified [332,412,418,419]. No evidence quality assessments were detailed. A high quality RCT demonstrated that a ten-day course of ciprofloxacin was superior to pivampicillin for clinical cure (80% vs. 60%) in men aged > 40 years [420]. Data from a large comparative case series suggested that young age and history of sexual activity are not sufficiently predictive of a sexually transmitted pathogen to guide antibiotic treatment in acute epididymitis [409].
Empiric antibiotic regimens from existing guidelines [332,412,418,419] and panel consensus:
For men with acute epididymitis at low risk of gonorrhoea (e.g. no urethritis, no urethral discharge) a single agent active against Enterobacterales and C. trachomatis (e.g. levofloxacin) or a combination of two agents (e.g. doxycycline and trimethoprim-sulfamethoxazol) to eradicate C. trachomatis and Enterobacterales should be used. Options are:
A. Levofloxacin 500 mg per os once daily for ten to fourteen days*
OR
B. Doxycycline 200 mg per os initial dose and then 100 mg twice daily for ten to fourteen days* plus trimethoprim-sulfamethoxazol 160/800mg twice daily for ten to fourteen days**
For biological men with likely gonorrhoeal acute epididymitis a combination regimen active against N. gonorrhoeae and C. trachomatis must be used such as:
A. Ceftriaxone 1000 mg intramuscularly or i.v. single dose plus doxycycline 200 mg per os initial dose by mouth and then 100 mg twice daily for ten to fourteen days*
- For non-sexually active men with acute epididymitis a single agent of sufficient dose and duration to eradicate Enterobacterales should be used. Appropriate option is a fluoroquinolone once daily (e.g. levofloxacin 500 mg) or trimethoprim-sulfamethoxazol 160/800mg per os twice daily for ten to fourteen days*.
*Depending upon pathogen identification and clinical response.
** A parenteral option will be required for men with severe infection requiring hospitalisation.
Surgical exploration may be required to drain abscesses or debride tissue. A comparative cohort study found that lack of separation of epididymis and testis on palpation and the presence of abscess on US may predict requirement for surgery following initial antibiotic treatment [421].
A cohort study found semen parameters may be impaired during epididymitis but recovered following successful treatment [422]. Comparative clinician cohort studies suggest adherence to guidelines for assessment and treatment of epididymitis is low, particularly by urologists compared to sexual health specialists [423] and by primary care physicians [424].
3.12.5. Screening
A large cohort screening study for carriage of C. trachomatis including a randomly selected group of 5,000 men of whom 1,033 were tested showed no benefit in terms of reduction in risk of epididymitis over nine years of observation [425].
3.12.6. Summary of evidence and recommendations for the diagnosis and treatment of acute infective epididymitis
| Summary of evidence | LE |
| In young sexually active patients, both sexually transmitted infections (STIs) and Enterobacterales have to be considered as aetiological agents. | 3 |
| In patients > 40 years, antibiotic therapy with ciprofloxacin is superior to pivmecillinam. | 1b |
| A negative sexual risk history does not exclude STIs in sexually active men. | 3 |
| Recommendations | Strength rating |
| Obtain a mid-stream urine and a first voided urine for pathogen identification by culture and nucleic acid amplification test. | Strong |
| Initially prescribe a single antibiotic or a combination of two antibiotics active against Chlamydia trachomatis and Enterobacterales in young sexually active men; in older men without sexual risk factors, only Enterobacterales have to be considered. | Strong |
| If gonorrhoeal infection is likely, give single dose ceftriaxone 1000 mg intramuscularly or intravenously* in addition to a course of an antibiotic active against Chlamydia trachomatis. | Strong |
| Adjust antibiotic agent when pathogen has been identified and adjust duration according to clinical response. | Weak |
| Follow national policies on reporting and tracing/treatment of contacts for sexually transmitted infections. | Strong |
* Despite the lack of RCTs, there is increasing evidence that intravenous treatment with ceftriaxone is safe and effective for the treatment of gonorrhoeal infections and avoids the discomfort of an intramuscular injection for patients [359]
Figure 2: Diagnostic and treatment algorithm for men with acute epididymitis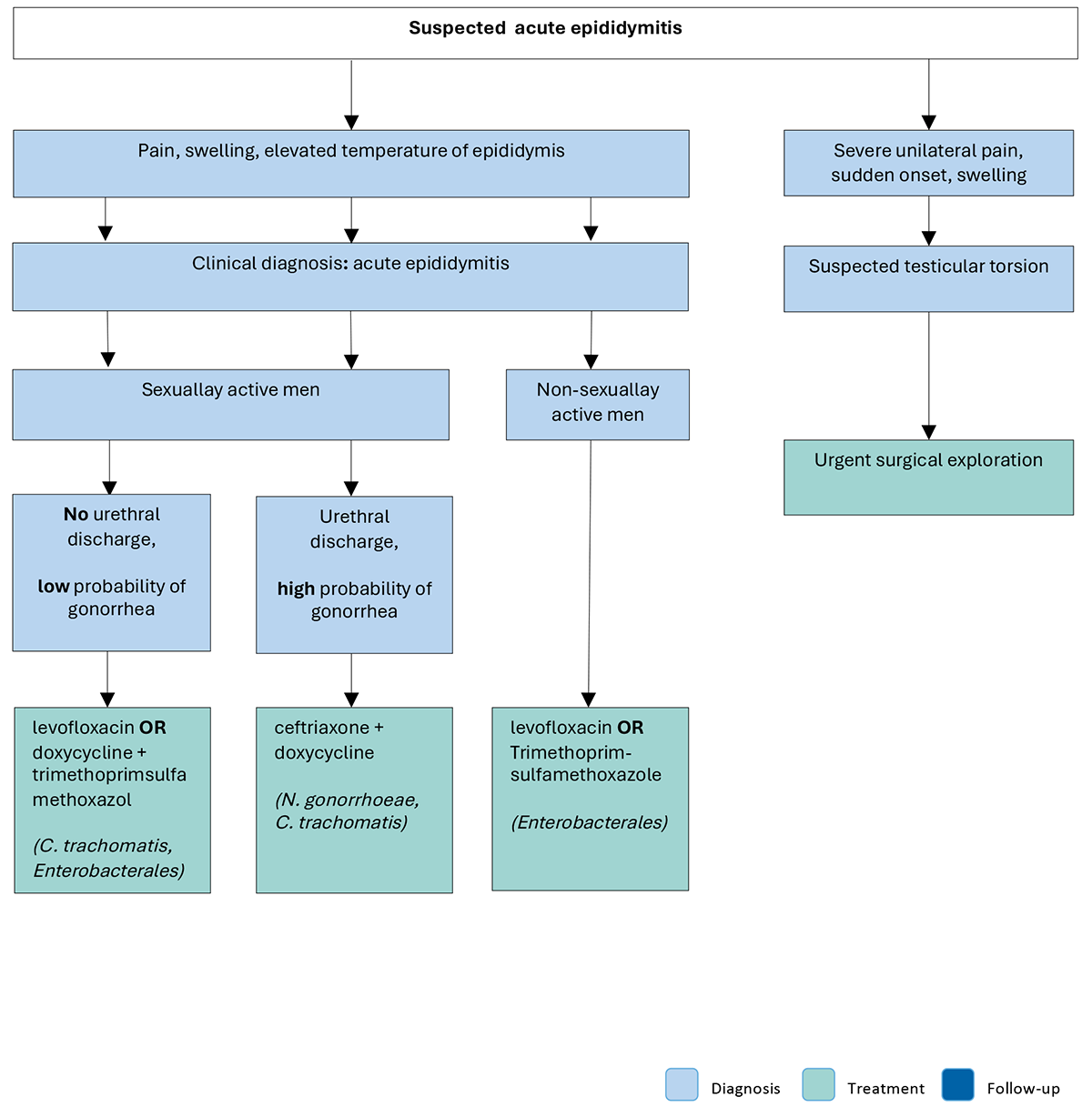
3.13. Fournier’s Gangrene (Necrotising fasciitis of the perineum and external genitalia)
3.13.1. Epidemiology, Aetiology and Pathophysiology
Fournier’s gangrene is an aggressive and frequently fatal polymicrobial soft tissue infection of the perineum, peri-anal region, and external genitalia [426]. It is an anatomical sub-category of necrotising fasciitis with which it shares a common aetiology and management pathway.
3.13.2. Diagnostic Evaluation
Typically, there is painful swelling of the scrotum or perineum with sepsis [426]. Examination shows small necrotic areas of skin with surrounding erythema and oedema. Crepitus on palpation and a foul-smelling exudate occurs with more advanced disease. Patient risk factors for occurrence and mortality include being immunocompromised, most commonly diabetes or malnutrition, recent urethral or perineal surgery, and high body mass index (BMI). In up to 40% of cases, the onset is more insidious with undiagnosed pain often resulting in delayed treatment [427]. A high index of suspicion and careful examination, particularly of obese patients, is required. Computed tomography or MRI can help define para-rectal involvement, suggesting the need for bowel diversion [426].
3.13.3. Disease Management
The degree of internal necrosis is usually vastly greater than suggested by external signs, and consequently, adequate, repeated surgical debridement with urinary diversion by suprapubic catheter is necessary to reduce mortality [426]. Consensus from case series suggests that surgical debridement should be early (< 24 hours) and complete, as delayed and/or inadequate surgery may result in higher mortality [426]. Immediate empiric parenteral antibiotic treatment should be given that covers all probable causative organisms and can penetrate inflammatory tissue. A suggested regime would comprise a broad-spectrum penicillin or third-generation cephalosporin, gentamicin and metronidazole or clindamycin [426]. This can then be refined, guided by microbiological culture.
3.13.4. Evidence Summary
A systematic literature search from 1980 to July 2017 was performed. From 640 references, one RCT [428], two systematic reviews [429,430], one narrative review [426], three registry studies [431-433], one prospective cohort study [434] and two retrospective comparative cohort studies with at least 25 patients [435,436] were selected. The three registry studies from the United States [431-433], found mortality rates of 10%, 7.5% and 5% from 650, 1,641 and 9,249 cases, respectively. Older age, diabetes and high BMI were associated with higher risk. A prospective cohort study showed that disease-specific severity scores did predict outcome, but were not superior to generic scoring systems for critical care [434]. The evidence questions addressed were:
- What is the best antimicrobial treatment strategy to reduce mortality?
- What is the best debridement and reconstruction strategy to reduce mortality and aid recovery?
- Are there any effective adjuvant treatments that improve outcome?
Concerning the evidence questions:
- A low-quality retrospective case series [435] with 168 patients found no significant difference in mortality between patients given ≤ 10 days of parenteral antibiotics (80 patients) and those given > 10 days (88 patients).
- A systematic review of wound closure techniques [430] found low-quality evidence from sixteen case series involving 425 male patients. They recommended primary or secondary wound closure for scrotal defects ≤ 50% with the use of flaps or skin grafts for defects involving > 50% of the scrotum or with extension outside the scrotum.
- A systematic review on the use of hyperbaric oxygen therapy [429] included three comparative case series and four other case series. All were retrospective and published prior to 2000. No consistent evidence of benefit was found; an RCT was advised. A more recent comparative case series [436] suggested benefit for use of hyperbaric oxygen therapy in sixteen patients compared to twelve cases without use of such therapy in terms of reduced mortality and fewer debridements (low-quality evidence). A low-quality RCT [428] with 30 patients found that use of honey-soaked dressings resulted in a shorter hospital stay (28 vs. 32 days) than dressing soaked with Edinburgh solution of lime (EUSOL). We found no evidence of benefit for use of negative-pressure (vacuum) wound therapy in Fournier’s gangrene.
3.13.5. Summary of evidence and recommendations for the disease management of Fournier’s Gangrene
| Summary of evidence | LE |
| Immediate empiric parenteral antibiotic treatment should be given that covers all probable causative organisms and can penetrate inflammatory tissue. | 3 |
| A systematic review of wound closure techniques recommended primary or secondary wound closure for scrotal defects ≤ 50% with the use of flaps or skin grafts for defects involving > 50% of the scrotum or with extension outside the scrotum. | 3 |
| No consistent evidence of benefit for hyperbaric oxygen therapy was found. | 3 |
| A low-quality RCT found that dressings soaked in honey resulted in a shorter hospital stay than dressing soaked with EUSOL. | 3 |
| No evidence of benefit for use of negative-pressure (vacuum) wound therapy in Fournier’s gan-grene was found. | 4 |
| Recommendations | Strength rating |
| Start treatment for Fournier’s gangrene with broad-spectrum antibiotics on presentation, with subsequent refinement according to culture and clinical response. | Strong |
| Commence repeated surgical debridement for Fournier’s gangrene within 24 hours of presentation. | Strong |
| Do not use adjunctive treatments for Fournier’s gangrene except in the context of clinical trials. | Weak |
Table 13: Suggested regimens for antimicrobial therapy for Fournier’s Gangrene of mixed microbiological aetiology adapted from [437]
| Antimicrobial | Dosage |
Piperacillin-tazobactam plus Vancomycin | 4.5 g q.i.d or t.i.d iv 15 mg/kg b.i.d |
| Imipenem-cilastatin | Standard dosage: 0.5 g iv q.i.d over 30 minutes High dosage: 1 g iv q.i.d over 30 minutes |
| Meropenem | 1 g t.i.d iv |
| Ertapenem | 1 g o.d |
| Gentamicin | 6-7 mg/kg iv q.d |
Cefotaxime plus metronidazole or clindamycin | 2 g q.i.d iv 500 mg q.i.d iv 600-900 mg t.i.d iv |
Cefotaxime plus fosfomycin plus metronidazole | 2 g q.i.d iv 5 g t.i.d iv 500 mg q.i.d iv |
iv = intravenous; b.i.d = twice daily; t.i.d = three times daily; q.i.d = four times daily, q.d = every day; o.d = once daily.
3.14. Management of Human papillomavirus in men
3.14.1. Epidemiology
Human papilloma virus (HPV) is one of the most frequently sexually transmitted viruses encompassing both oncogenic (low- and high-risk variants) and non-oncogenic viruses. Human papilloma virus 16 is the most common oncogenic variant, detected in 20% of all HPV cases [438]. A recent meta-analysis revealed a prevalence of 49% of any type of HPV and 35% of high-risk HPV in men [439]. Similar to the female genital tract, half of all HPV infections in the male genital tract are co-infections (≥ 2 HPV strains) [440].
Human papilloma virus presence is dependent on study setting. In men attending urological clinics HPV was detected in 6% of urine samples [441]. A meta-analysis reported seminal HPV in 4.5-15.2% of patients resulting in seminal HPV being associated with decreased male fertility [438]. A cross sectional study of 430 men presenting for fertility treatment detected HPV in 14.9% of semen samples [442]. The presence of HPV in semen was not associated with impaired semen quality [442]. However, another systematic review reported a possible association between HPV and altered semen parameters, and in women possible miscarriage or premature rupture of the membrane during pregnancy [443]. HPV6 and/or 11 were the most common genotypes detected in an observational study of anogenital warts, whilst HPV16 is correlated with severity of anal cytology [444]. The incidence of non-oncogenic HPV infection has been shown to be higher in men than women [445]. In males, approximately 33% of penile cancers and up to 90% of anal cancers are attributed to high-risk HPV infections, primarily with HPV16 [446]. The EAU Penial Cancer Guidelines published a comprehensive update in March 2022 including the results of two systematic reviews on HPV and penile cancer. Oral HPV is associated with oropharyngeal carcinomas approximately 22.4%, 4.4% and 3.5% of oral cavity, oropharynx and larynx cancers, respectively are attributed to HPV [446]. Systematic reviews have reported prevalence rates of oral HPV from 5.5-7.7%, with HPV16 present in 1-1.4% of patients [447,448].
3.14.2. Risk factors
Risk factors for HPV infection include early age of first sexual intercourse, sexual promiscuity, higher frequency of sexual intercourse, smoking and poor immune function [449-453]. Incidence and prevalence of overall HPV was considerably higher in MSM compared to heterosexuals [447,450]. Overall, the prevalence of HPV in different sites seems to be higher in young, sexual-active adults compared to other population groups [449]. Stable sexual habits, circumcision and condom use are protective factors against HPV [439,453-457]. Added risk factors of oral HPV infection are alcohol consumption, poor oral hygiene and sexual behaviours (oral and vaginal) [447,449]. Positive HIV status, phimosis, and HPV status of the partner have also been associated with anogenital HPV status and decreased clearance in a number of studies [454].
3.14.3. Transmission
Human papilloma virus typically spreads by sustained direct skin-to-skin or mucosal contact, with vaginal, oral and anal sex being the most common transmission route [451]. In addition, HPV has been found on surfaces in medical settings and public environments raising the possibility of object-to-skin/mucosa transmission [458]. Further studies on non-sexual and non-penetrative sexual transmission are needed to understand the complexity of HPV transmission. HPV transmission may also be influenced by genotype, with a higher incidence of HPV51 and HPV52 and a high prevalence of HPV16 and HPV18 in the general and high-risk male population [451].
3.14.4. Clearance
Human papilloma virus time-to-clearance ranges from 1.3 to 42.1 months [459]. Clearance may be influenced by HPV genotype, patients’ characteristics and affected body site [450,454,459]. Human papilloma virus 16 has the highest incidence of high-risk HPV variants and has the lowest clearance across sites [454].
3.14.5. Diagnosis
There is currently no approved test for HPV in men. Routine testing to check for HPV or HPV-related disease in men is not recommended. A physical examination to identify HPV lesions should be carried out. An acetic acid test to diagnose sub-clinical HPV lesions may be performed. If the diagnosis is uncertain or there is a suspicion of cancer a biopsy should be carried out. Intra-urethral condylomas are relatively uncommon and are usually limited to the distal urethral meatus [460,461]. Urethrocystoscopy may be used to diagnose the presence of intra-urethral or bladder warts [461]; however, there is no high-level evidence for the use of invasive diagnostic tools for localisation of intra-urethral HPV. For detailed recommendations on the diagnosis of anogenital warts please refer to the IUSTI‐European guideline for the management of anogenital warts [462].
3.14.6. Treatment of HPV related diseases
Approximately 90% of HPV infections do not cause any problems and are cleared by the body within two years. However, treatment is required when HPV infection manifests as anogenital warts to prevent the transmission of HPV-associated anogenital infection and to minimise the discomfort caused to patients [462]. Of the treatment options available, only surgical treatment has a primary clearance rate approaching 100%.
3.14.6.1. Treatments suitable for self-application
Patient-applied treatments include podophyllotoxin, salicylic acid, imiquimod, polyphenon E, 5-fluoracil and potassium hydroxide [462]. Imiquimod 5% cream showed a total clearance of external genital or perianal warts in 50% of immunocompetent patients [463] as well as in HIV positive patients successfully treated with highly active antiretroviral therapy [464]. A Cochrane review of published RCTs found imiquimod to be superior to placebo in achieving complete clearance of warts (RR: 4.03, 95% CI: 2.03–7.99) [465]. The recommended treatment schedule is imiquimod 5% cream applied to all external warts overnight three times each week for sixteen weeks [462]. In an RCT involving 502 patients with genital and/or perianal warts sinecatechins 15% and 10% showed a complete clearance of all baseline and newly occurring warts in 57.2% and 56.3% of patients, respectively vs. 33.7% for placebo [466]. In addition, sinecatechins 10% has been shown to be associated with lower short-term recurrence rates when used as sequential therapy after laser CO2 ablative therapy [467]. Sinecatechins is applied three times daily until complete clearance, or for up to sixteen weeks. Clearance rates of 36–83% for podophyllotoxin solution and 43–70% for podophyllotoxin cream have been reported [462]. A systematic review and meta‐analysis confirmed the effectiveness of podophyllotoxin 0.5% solution relative to placebo (RR: 19.86, 95% CI: 3.88–101.65) [468]. Podophyllotoxin is self‐applied to lesions twice daily for three days, followed by four rest days, for up to four or five weeks. An RCT has also shown potassium hydroxide 5% to be an effective, safe, and low-cost treatment modality for genital warts in men [469].
3.14.6.2. Physician-administered treatment
Physician-administered treatments included cryotherapy (79-88% clearance rate; 25-39% recurrence rate), surgical treatment (61-94% clearance rate), including excision, electrosurgery, electrocautery and laser therapy (75% clearance rate) [470,471]. Physician-administered therapies are associated with close to 100% clearance rates, but they are also associated with high rates of recurrence as they often fail to eliminate invisible HPV-infected lesions [470,471]. No data about the superiority of one treatment over another are available. However, among all interventions evaluated in a recent systematic review and network meta-analysis, surgical excision appeared to be the most effective treatment at minimising risk of recurrence [472].
3.14.6.3. Summary of evidence and recommendations for the treatment of anogenital warts
| Summary of evidence | LE |
| A Cochrane review of published RCTs found imiquimod to be superior to placebo in achieving complete clearance of warts. | 1b |
| In an RCT, sinecatechins 15% and 10% showed a complete clearance of all baseline and newly occurring warts in 57.2% and 56.3% of patients, respectively vs. 33.7% for placebo. | 1b |
| A systematic review and meta‐analysis confirmed the effectiveness of podophyllotoxin 0.5% solution relative to placebo. | 1b |
| A systematic review and meta-analysis reported that among all physician-applied therapy, surgical excision seemed to be the most effective at minimising risk of recurrence. | 1a |
| Recommendations | Strength rating |
| Use self-administered imiquimod 5% cream applied to all external warts overnight three times each week for sixteen weeks for the treatment of anogenital warts. | Strong |
| Use self-administered sinecatechins 15% or 10% applied to all external warts three times daily until complete clearance, or for up to sixteen weeks for the treat-ment of anogenital warts. | Strong |
| Use self-administered podophyllotoxin 0.5% self‐applied to lesions twice daily for three days, followed by four rest days, for up to four or five weeks for the treatment of anogenital warts. | Strong |
| Use cryotherapy or surgical treatment (excision, electrosurgery, electrocautery and laser therapy) to treat anogenital warts based on an informed discussion with the patient. | Strong |
3.14.7. Circumcision for reduction of HPV prevalence
Male circumcision is a simple surgical procedure which has been shown to reduce the incidence of STIs including HIV, syphilis and HSV-2 [473]. Two systematic reviews and meta-analyses, showed an inverse association between male circumcision and genital HPV prevalence in men [457,459]. It has been suggested that male circumcision could be considered as an additional one-time preventative intervention likely to reduce the burden of HPV-related diseases in both men and women, particularly among those countries in which HPV vaccination programs and cervical screening are not available [459].
| Summary of evidence | LE |
| Two systematic reviews and meta-analyses, showed an inverse association between male circumcision and genital HPV prevalence in men. | 1a |
| Recommendation | Strength rating |
| Discuss male circumcision with patients as an additional one-time preventative intervention for HPV-related diseases. | Strong |
3.14.8. Therapeutic vaccination
Three different vaccines against HPV have been licensed to date, but routine vaccination of males is currently implemented in only a few countries including Australia, Canada, the USA and Austria. The aim of male vaccination is to reduce the rate of anal and penile cancers as well as head and neck cancers [446,474].
A systematic review including a total of 5,294 patients reported vaccine efficacy against persisting (at least six months) anogenital HPV16 infections of 46.9% (28.6-60.8%) and against persisting oral infections of 88% (2–98%). A vaccine efficacy of 61.9% (21.4–82.8%) and 46.8% (20-77.9%) was observed against anal intraepithelial neoplasia grade 2 and 3 lesions, respectively [446]. The systematic review reported no meaningful estimates on vaccine efficacy against penile intraepithelial neoplasia grade 2 or 3, and no data were identified for anal, penile or head and neck squamous cell cancers [446].
A phase III clinical trial including 180 male patients evaluated the potential of MVA E2 recombinant vaccinia virus to treat intraepithelial lesions associated with papillomavirus infection [475]. The study showed promising results in terms of immune system stimulation against HPV lesions as well as regression in intraepithelial lesions.
| Summary of evidence | LE |
| The role of therapeutic HPV vaccination in males in terms of effectiveness and safety is limited by the small number of relevant studies. | 2 |
| Therapeutic HPV vaccination in males is moderately effective against persistent anogenital HPV16 infection [(46.9% (28.6-60.8%)] and high-grade anal intraepithelial lesions [grade 2: 61.9% (21.4–82.8%); grade 3: 46.8% (20-77.9%)]. | 1b |
| Recommendation | Strength rating |
| Offer HPV vaccine to males after surgical removal of high-grade anal intraepithelial neoplasia. | Weak |
3.14.9. Prophylactic vaccination
A systematic review and meta-analysis reported that vaccination is moderately effective against genital HPV-related diseases irrespective of an individual’s HPV status; however, higher vaccine efficacy was observed in HPV-naïve males [446]. Supporting the early vaccination of boys with the goal of establishing optimal vaccine-induced protection before the onset of sexual activity [446]. An RCT including 1,124 demonstrated high efficacy of the quadrivalent HPV vaccine vs. placebo against HPV6/11/16/18-related persistent infections [476]. Furthermore, the vaccine elicited a robust immune response and was well tolerated with mild vaccination-related adverse events e.g. injection-site pain and swelling [476]. In addition, a Cochrane review, demonstrated that the quadrivalent HPV vaccine appears to be effective in the prevention of external genital lesions and genital warts in males [477].
Despite the fact quadrivalent HPV vaccines were approved for use in young adult males in 2010, vaccination rates have remained low at 10-15% [478]. Barriers to uptake in this patient group include lack of awareness about HPV vaccines and HPV-related diseases, concerns about vaccine safety and efficacy, economic/cost issues related to vaccine uptake, underestimation of HPV infection risks and sexual activity [478]. Health care professionals should provide easily understood and accessible communication resources regarding these issues, in order to educate young adult males and their families on the importance of HPV vaccination to reduce the incidence of certain cancers in the later life [478,479].
| Summary of evidence | LE |
| HPV vaccine is effective in the prevention of external genital lesions and genital warts in males. | 1a |
| HPV vaccination is moderately effective against genital HPV-related diseases irrespective of a individual’s HPV status; however, higher vaccine efficacy was observed in HPV-naïve males. | 1a |
| A systematic review of HPV vaccination barriers among adolescent and young adult males identified a number of barriers to vaccine uptake including fear of side-effects, limited HPV awareness, financial costs and changes in sexual activity. | 1b |
| An intervention study to evaluate whether electronic messaging can increase human papillomavirus vaccine completion and knowledge among college students concluded that intervention increased knowledge but not vaccine completion. | 2b |
| Recommendations | Strength rating |
| Offer early HPV vaccination to boys with the goal of establishing optimal vaccine-induced protection before the onset of sexual activity. | Strong |
| Apply diverse communication strategies in order to improve HPV vaccination knowledge in young adult males. | Strong |
Figure 3: Diagnostic and treatment algorithm for the management of HPV in men
3.15. Herpes Simplex Virus
3.15.1. Epidemiology, Aetiology and Pathogenesis
Genital herpes (GH) is a highly prevalent sexually transmitted, lifelong viral infection caused by the HSV, which exists in two types: HSV-1 and HSV-2. Genital herpes accounts for up to 60% of genital ulcers, with approximately one-third caused by HSV-1 and two-thirds by HSV-2 [480]. Co-infection with both virus types occurs in up to 44.7% of patients [481]. The estimated seroprevalence of HSV-1 among healthy adults is 84.8%, while for HSV-2 it ranges from 10-12.44% [482-485]. Both HSV-1 and 2 result in chronic infection, with frequent reactivations and subclinical shedding, leading to a high risk of transmission to sexual partners.
Risk factors for GH include high sexual promiscuity, a large number of lifetime sexual partners, rural residence, and unprotected sex [486,487]. Increased prevalence is seen in intermediate- and high-risk subgroups, such as men who have sex with men (MSM), HIV-infected patients, those living with HIV-infected partners, STDI clinic attendees and transgender individuals [483-485].
3.15.2. Diagnostic
Genital herpes clinical diagnosis can be challenging, as ulcerative lesions may be absent during evaluation. When present, the lesions are typically painful, erythematous, vesicular, and recurrent. Less common presentations include nodular, hypertrophic, verrucous, vegetative or exophytic lesions [488]. Whenever possible, GH diagnosis should be confirmed by laboratory testing [489]. Swabs should be taken from the lesion for type-specific virus identification using PCR or culture [488]. PCR detection of HSV DNA is rapid and more sensitive than traditional culture and immunoassay methods [490-492]. While PCR accuracy may vary, most tests available exhibit sensitivity and specificity > 90% [493,494]. HSV serological assays, ideally using the Western blot technique, can detect antibodies against multiple HSV-1 and HSV-2 antigens, including glycoprotein G [495-497]. However, the panel does not recommend serological testing for diagnosing localised genital HSV infections. During the last few years rapid point-of-care tests for serological HSV diagnosis have become available [498,499]. Although these methods show high sensitivity and specificity, they do not provide quantitative results, making them less useful than laboratory tests. Currently, there is insufficient evidence to recommend these rapid tests as diagnostic standard in clinical practice. Serological testing is primarily indicated for detecting past HSV infections and is not recommended for diagnosing acute or recent infections, where virus detection from active lesions remains the preferred method.
3.15.3. Disease Management
3.15.3.1. Systemic Treatment
Antiviral drugs used to treat HSV include acyclovir, valacyclovir, pritelivir [500], amenamevir [501,502], and inosine pranobex [503]. Short-term recurrence rates are high, ranging from 27-48% [503]. Antiviral therapies reduce both the frequency and viral load during shedding [500,504,505]. With treatment, the rate of viral shedding decreases from 16% to approximately 5% [500], and the presence of visible lesions is reduced from 9% to about 1.2% [500]. While the optimal duration of treatment is not clearly defined, beneficial effects such as the reduction of ulcerative lesions and pain can be observed even after a single dose [502].
3.15.3.2. Topical Treatment
Topical treatments play a limited role in managing GH and their use is not recommended. Immunomodulation with resiquimod showed no improvement in outcomes and increased the incidence of local side effects [506]. CS21 (oxygenated glycerol triesters-based barrier genital gel) improved symptoms and slowed disease progression compared to both placebo and topical acyclovir [507]. Topical acyclovir did not outperform placebo in terms of disease progression [507].
3.15.3.3. Vaccination
Various studies have evaluated the efficacy of therapeutic vaccines for HSV treatment and transmission prevention [508-513]. However, there is currently insufficient evidence to support their use in clinical practise.
3.15.3.4. Surgical Management
The impact of surgical interventions in patients with HSV remains unclear. Following circumcision, there may be an increase in viral shedding in the initial weeks due to inflammation [514]. Photodynamic therapy using a photosensitizer has shown potential in a small trial for treating recurrent lesions, however, further research is needed before it can be recommended in clinical practice [515].
3.15.4. Prevention, Screening & Contact Tracing, Follow-up
Glandular exposure through circumcision has been reported to prevent HSV infections [516]. A study on the pericoital application of tenofovir gel showed a reduced HSV-2 acquisition in women [517]. However, there is insufficient data to recommend any form of pre-exposure prophylaxis with HSV antiviral medications for prevention, and it should not be offered as a preventive strategy.
In accordance with most national and international guidelines, the panel does not recommend routine herpes testing for asymptomatic individuals. Screening is recommended only in the presence of genital symptoms potentially related to HSV or if a sexual partner has GH.
Follow-up testing is not indicated, except when there are recurrent signs and symptoms. Counselling of individuals with GH and their sexual partners is of mandatory importance to prevent transmission and cope with the infection.
| Level of evidence | LE |
| Genital herpes accounts for up to 60% of genital ulcers with approximately one-third caused by HSV-1 and two-thirds by HSV-2. | 2b |
| Detection of HSV DNA via PCR is rapid and more sensitive than traditional culture and immunoassay methods. | 2a |
| Antiviral therapies reduce both the frequency and viral load during viral shedding. | 1b |
| Viral shedding may temporarily increase in the weeks following circumcision due to inflammation. | 3 |
| Topical antiviral therapy does not improve clinical outcomes of HSV patients, increase local side effects, but can reduce the local viral load. | 1b |
| Recommendations | Strength rating |
| Obtain a comprehensive medical history, including history of previous sexual contacts from all patients presenting with genital ulcers potentially related to HSV. | Strong |
| Confirm the diagnosis with a clinical swab and type-specific virologic testing, such as PCR or culture, from the lesion. | Strong |
| Treat the first clinical episode of genital HSV infection. | Strong |
Table 14: Treatment regimens for genital HSV infection
| Antimicrobials | Dosage |
| Recommended therapy and dose for first clinical episode HSV | |
| Aciclovir | 400 mg orally t.i.d for 10 days OR 200 mg orally 5 times daily for 10 days. |
| Valaciclovir | 500 mg orally b.i.d for 10 days. |
| Recommended therapy and dose for recurrent genital HSV | |
| Aciclovir | 400 mg orally t.i.d for 5 days OR 800 mg b.i.d for 5 days OR 800 mg t.i.d for 2 days. |
| Valaciclovir | 500 mg orally b.i.d for 3 days. |
b.i.d. = twice daily; t.i.d = three times daily; q.d = every day; o.d = once daily
3.16. Genitourinary Tuberculosis
3.16.1. Epidemiology, Aetiology and Pathophysiology
An estimated 246,000 new and relapse tuberculosis (TB) cases occurred in the WHO European Region in 2019, with 49,752 of these cases occurring within the 31 countries comprising the European Union (EU)/European Economic Area (EEA) region [518]. An estimated 12.0% of incident TB cases in 2019 were co-infected with HIV. Extrapulmonary TB was notified on average for 16.6% of all incident TB cases in the Region. Eleven countries reported more than 30% of their TB cases having extrapulmonary localisation. The proportion of TB that is extrapulmonary is significantly greater among migrants than non-migrants. Genitourinary tuberculosis (GUTB) accounted for 4.6% of extrapulmonary TB cases in the EU between 1997-2017 [519]. Tuberculosis is an infectious disease caused by a group of Mycobacterium species called the Mycobacterium tuberculosis complex (MBTC) [520]. Genitourinary TB can affect all genitourinary organs and is almost always secondary due to the hematogenous spread of chronic latent TB infection (LTBI) [521]. Risk factors include primary and LTBI, diabetes, old age, low BMI, oncological comorbidities, immune suppression (including HIV), renal failure and poor socioeconomic living conditions. The risk of reactivation is estimated to be up to 15% during one’s lifetime [522]. The WHO recommend either a tuberculin skin test (TST) or interferon-gamma release assay (IGRA) for the diagnosis LTBI [523].
3.16.2. Diagnosis
The diagnosis of GUTB is challenging as no single diagnostic test exists. Diagnosis relies on a high suspicion of infection based on patient history; microbiological, molecular and histological testing; and imaging findings. Patients generally present with non-specific urological complaints for which no obvious cause is identified including haematuria, increased urinary frequency, difficulty voiding, abdominal, lumbar and suprapubic pain, and in female patients menstrual irregularities and pelvic pain. Patients may also present for infertility issues; however, infertility and TB will not be addressed in detail in this text.
3.16.2.1. Smear Microscopy
Smear microscopy is a simple and cost-effective way of detecting the presence of acid-fast bacilli (AFB) in urine samples, semen, tissue specimens, pus, or discharged or prostatic massage fluid, through microscopic examination using Ziehl–Neelsen or auramine staining [524,525]. A major limitation of smear microscopy is its low sensitivity (ranging from 0-25%) in urine [526,527].
3.16.2.2. Culture
The culture-based method (both solid and liquid media) for biological specimens is the reference standard for M. tuberculosis isolation from biological samples. Three midstream first-void urine samples, on consecutive days, are recommended for TB culture [525]. A disadvantage of culture-based methods is the long incubation period needed for results at least nine to ten days for positive results and six weeks to be considered negative as well as the need for highly equipped laboratories. In addition, studies have reported high specificities of 92–100% but low sensitivities 23.3–30% for urine culture in renal TB specimens [528,529].
3.16.2.3. Nucleic Acid Amplification Tests
In recent years, NAATs have been introduced in the diagnostic pathway of TB, to overcome the limits of early and rapid diagnosis and of drug susceptibility testing. In 2021 the WHO issued an update to its guidelines for the rapid diagnosis of TB in which they made a conditional recommendation that in patients with signs and symptoms of extrapulmonary TB, Xpert MTB/RIF may be used as an the initial diagnostic test [530]. Xpert MTB/RIF pooled sensitivity and specificity were 84.7% (70.8 to 93.1) and 97.3% (91.0 to 99.2) for the diagnosis of genitourinary TB [531]. The 2021 WHO guidelines also contain a number a recommendations for additional PCR testing systems as well as moderate complexity automated NAATs [530].
Note: A number of other diagnostic tests are currently under investigation by the WHO but cannot be recommended for the diagnosis of GUTB at this time.
3.16.2.4. Imaging
Imaging modalities aid in the localisation of the foci of infection in GUTB and in the assessment of the extent of the damage to the genito-urinary system. Imaging techniques for the diagnosis of GUTB have a sensitivity of approx. 90% [532]. However, the quality of the evidence available for diagnostic imaging of TB is low to very-low and further studies are required to allow the Panel to make recommendations on this topic.
Ultrasound is a cost-effective and non-invasive imaging modality that has been shown to be effective for the diagnosis of testicular, epididymal and vas deferens TB [533-537]. Ultrasound examination may also allow for the identification of parenchymal masses, cavities, mucosal thickening of the collecting system and bladder, stenosis and consecutive obstruction of the collecting system, vesicoureteral reflux, and calcifications [538]. In female GUTB patients US may identify ovarian masses, intrauterine thickening and calcifications [539].
Intravenous urography aids in the identification of renal and ureteral TB, but lacks specificity. Approximately 10-15% of patients may have normal findings on IVU [540,541]. The most common findings on IVU are hydrocalycosis, hydronephrosis or hydroureter due to stricture, autonephrectomy and urinary calcifications [542-544].
In recent years CT and MRI have largely replaced IUV. The most common findings on CT are parenchymal scarring, hydrocalycosis, hydronephrosis or hydroureter due to stricture, and thickening of the renal pelvis, ureter and bladder walls [542-544]. In TB of the seminal vesicles and vas deferens CT imaging can show enlarged heterogeneously enhancing seminal vesicles with possible wall thickening, contraction, and intraluminal or wall calcifications [545,546]. Prostate TB appears as a low attenuating and marginally enhancing cystic mass, which is indistinguishable from a non-TB prostatic abscess [547]. In female GUTB the fallopian tubes are most frequently affected area and present with enlargement, hydrosalpinx, pyosalpinx, and wall thickening, with calcification on CT [548].
Magnetic resonance imaging has low sensitivity for the diagnosis of GUTB in the early stages of the infection [549]. As an imaging modality MRI is useful in patients in whom CT is contraindicated, including patients with renal failure or contrast hypersensitivity reactions or those who wish to avoid exposure to radiation. Renal and ureteral abnormalities are comparable to those described for CT findings and must be distinguished from acute pyelonephritis [539,550]. Epididymitis and testicular TB appears as a diffusely enlarged epididymis or testis with heterogeneous high T2 signal due to fibrosis and calcification [546]. Multiparametric MRI of the prostate distinguishes between the nodular or diffuse patterns of prostate TB [551].
Female GUTB has a wide range of appearances on HSG affecting the fallopian tubes, endometrium and uterus [552,553]. Tubal obstruction is the most common finding with HSG [553]. In addition, deformity of the uterine cavity can be observed, such as a T-shaped and Dwarfed uterus, resulting from abnormal scaring and fibrosis [552]. As the disease progresses this process can potentially lead to a complete obliteration of the uterine cavity referred to as Netter syndrome [554].
3.16.3. Medical Treatment
The WHO recommends a daily six month regimen for treatment of newly diagnosed extrapulmonary TB, including an intensive phase of two months with isoniazid, rifampicin, pyrazinamide, and ethambutol, followed by a continuation phase of four months with isoniazid and rifampicin [555]. For the treatment of multi-drug resistant (MDR) TB (i.e. resistance to rifampicin and isoniazid) an individualised treatment regime should be applied with at least five effective tuberculosis medicines during the intensive phase, including pyrazinamide and four core second-line tuberculosis medicines [556].
3.16.4. Surgical treatment
Combination drug therapy is the first-line treatment for GUTB. However, in more than 50% of patients ablative, endoscopic or reconstructive surgery is required due to the destructive nature of the infection coupled with a delay in initial diagnosis [557-559]. In 26.9% of cases of diagnosed GUTB there is a non-functioning unilateral kidney and in 7.4%, renal failure [559].
In the largest observational study of 4,288 GUTB patients a total of 2,364 different surgical procedures were carried out of which 948 were reconstructive [560]. In a retrospective series of 241 patients who underwent surgery for GUTB, a total of 128 reconstructive procedures were done in which 30.29% of patients had bladder augmentation [561]. A retrospective single-centre study of 128 patients reported renal units in the reconstruction group had 5.44-fold longer survival than the permanent diversion group suggesting that when feasible renal reconstruction may be better for renal function preservation [562]. Reconstructive surgery may include augmentation cystoplasty, uretero-ureterostomy, ureteroneocystostomy, ureteral reimplant, pyeloplasty, ureterocalicostomy and ileal ureter or external diversion, where indicated [563].
There is limited evidence with regard to the optimum surgical approach. Minimally invasive options, have been reported as feasible and safe strategies, comparable to open surgery [564-568]. In addition, the optimal timing for surgery is controversial. A delay of two to six weeks up to nine months after the initiation of medical treatment has been proposed to allow for a reduction in active inflammation and stabilisation of the TB lesions [549].
Due to lack of high-quality evidence for surgical treatment of GUTB, the Panel is unable to give a recommendation on surgical treatment at this point in time. Patients with GUTB should be assessed on an individualised bases, and the decision to operate should be made depending on the location, extent of disease progression and damage to the genitourinary system.
3.16.5. Summary of evidence and recommendations for the diagnosis and treatment of GUTB
| Summary of evidence | LE |
| The risk of reactivation of latent TB is estimated to be 15% in an individual’s lifetime. | 2a |
| Smear microscopy for acid-fast bacilli has a low sensitivity in urine ranging from 0-25%. | 2a |
| Studies have reported high specificities of 92–100%, but low sensitivities of 23.3–30% for urine culture in renal TB specimens. | 2a |
| Xpert MTB/RIF pooled sensitivity and specificity were 84.7% (70.8 to 93.1) and 97.3% (91.0 to 99.2) for the diagnosis of GUTB. | 1b |
| Standard six month anti-tuberculous drug regimens are effective in all forms of TB (pulmonary and extrapulmonary). | 1a |
| There is limited evidence with regard to the optimum surgical approach and timing of surgery in GUTB patients. | 3 |
| Recommendations | Strength rating |
| Diagnosis | |
| Take a full medical history including history of previous tuberculosis infection (pulmonary and extrapulmonary) form all patients presenting with persistent non-specific genitourinary symptoms and no identifiable cause. | Strong |
| Perform smear microscopy on urine, semen, tissue specimens, discharged or prostatic massage fluid using Ziehl–Neelsen (ZN) or auramine staining in patients with suspected genitourinary tuberculosis (GUTB). | Weak |
| Perform acid-fact bacilli culture on three midstream first-void urine samples, on three consecutive days for M. tuberculosis isolation in patients with suspected GUTB. | Strong |
| Use a recommended PCR test systems in addition to microbiological reference standard (MRS) in urine specimens as a diagnostic test in patients with signs and symptoms of GUTB. | Weak |
| Use imaging modalities in combination with culture and/or PCR to aid in the diagnosis of GUTB and to assess the location and extent of damage to the genitourinary system. | Weak |
| Treatment | |
| Use medical treatment as first-line treatment for GUTB. | Strong |
| Use a daily six-month regimen for treatment of newly diagnosed GUTB, this should include an intensive phase of two months with isoniazid, rifampicin, pyrazinamide and ethambutol. Followed by a continuation phase of four-months with isoniazid and rifampicin. | Strong |
| Treat multi-drug resistant TB with an individualised treatment regime including at least five effective tuberculosis medicines during the intensive phase, including pyrazinamide and four core second-line tuberculosis medicines. | Strong |
Table 15: Treatment regimens for newly diagnosed GUTB and MDR-TB [556]
| Antimicrobials | Dosage |
| Six month regimen for treatment of newly diagnosed GUTB | |
| Intensive two month phase | |
| Isoniazid | 5 mg/kg q.d; max daily dosage 300 mg |
| Rifampicin | 10 mg/kg q.d; max daily dosage 600 mg |
| Pyrazinamide | 25 mg/kg q.d; max daily dosage 2000 mg |
| Ethambutol | 15–20 mg/kg q.d; max daily dosage ranging from 800 mg to 1600 mg depending on body weight |
| Continuation four month phase | |
| Isoniazid | 5 mg/kg q.d; max daily dosage 300 mg |
| Rifampicin | 10 mg/kg q.d; max daily dosage 600 mg |
| Treatment regimen for multi-drug resistant TB | |
| Treat multi-drug resistant TB with an individualised treatment regime including at least five effective tuberculosis medicines during the intensive phase, including pyrazinamide and four core second-line tuberculosis medicines*. | |
Group A Fluoroquinolones | Levofloxacin, Moxifloxacin and Gatifloxacin |
Group B Second-line injectables | Amikacin, Capreomycin, Kanamycin and Streptomycin** |
Group C Other second-line agents | Ethionamide/ Prothionamide, Cycloserine/Terizidone, Linezolid and Clofazimine |
Group D Add-on agents (not part of the core MDR-TB regime) | D1: Pyrazinamide, Ethambutol, and High-dose isoniazid D2: Bedaquiline and Delamamid D3: p-aminosalicylic acid, Imipenem-cilastatin, Meropenem, Amoxicillin-clavulanate and Thioacetazone*** |
b.i.d = twice daily; t.i.d = three times daily; q.d = every day; o.d = once daily
* Drugs should be chosen as follows: 1 from group A, 1 from group B, and at least 2 from group C. If the minimum number of five TB medicines cannot be composed from drugs included in Groups A to C, an agent from group D2 and other agents from group D3 may be added to bring the total to five [556]
**Streptomycin can substitute other injectable drugs if none of these agents can be used and if the strain is shown not to be resistant [556]
**Thioacetazone should not be used if the patient is HIV seropositive [556]
3.17. Peri-Procedural Antibiotic Prophylaxis
3.17.1. General Principles
3.17.1.1. Definition of infectious complications
The European Centre for Disease Prevention and Control (ECDC) and the CDC have both presented similar definitions recommended for the evaluation of infectious complications [569,570].
3.17.1.2. Non-antibiotic measures for asepsis
There are a number of non-antibiotic measures designed to reduce the risk of surgical site infection (SSI), many are historically part of the routine of surgery. The effectiveness of measures tested by RCTs are summarised in systematic reviews conducted by the Cochrane Wounds Group (http://wounds.cochrane.org/news/reviews). Urological surgeons and the institutions in which they work, should consider and monitor the maintenance of an aseptic environment to reduce the risk of infection from pathogens within patients (microbiome) and from outside the patient (nosocomial/healthcare-associated). This should include the use of correct methods of instrument cleaning and sterilisation, frequent and thorough cleaning of operating rooms and recovery areas and thorough disinfection of any contamination. The surgical team should prepare for surgery by effective hand washing [571], donning of appropriate protective clothing, and maintenance of asepsis. These measures should continue as required in recovery and ward areas.
Patients should be encouraged to shower pre-operatively, but use of chlorhexidine soap does not appear to be beneficial [572]. Although evidence quality is low, any required hair removal appears best done by clipping, rather than shaving, just prior to incision [573]. Mechanical bowel preparation should not be used as evidence review suggests harm not benefit [574,575]. There is some weak evidence that skin preparation using alcoholic solutions or chlorhexidine result in a lower rate of SSI than iodine solutions [576]. Studies on the use of plastic adherent drapes showed no evidence of benefit in reducing SSI [577].
3.17.1.3. Detection of bacteriuria prior to urological procedures
Identifying bacteriuria prior to diagnostic and therapeutic procedures aims to reduce the risk of infectious complications by controlling any pre-operative detected bacteriuria and to optimise antimicrobial coverage in conjunction with the procedure. A systematic review of the evidence identified eighteen studies comparing the diagnostic accuracy of different index tests (dipstick, automated microscopy, dipslide culture and flow cytometry), with urine culture as the reference standard [578]. The systematic review concluded that none of the alternative urinary investigations for the diagnosis of bacteriuria in adult patients prior to urological interventions can currently be recommended as an alternative to urine culture [578].
3.17.1.4. Choice of agent
Urologists should have knowledge of local pathogen prevalence for each type of procedure, their antibiotic susceptibility profiles and virulence in order to establish written local guidelines. These guidelines should cover the five modalities identified by the ECDC following a systematic review of the literature [579]. The agent should ideally not be one that may be required for treatment of infection. When risk of skin wound infection is low or absent, an aminoglycoside (gentamicin) should provide cover against likely uropathogens provided the eGFR is > 20 mL/min; second generation cephalosporins are an alternative [580]. Recent urine culture results including presence of any multi-resistant organisms, drug allergy, history of C. difficile associated diarrhoea, recent antibiotic exposure, evidence of symptomatic infection pre-procedure and serum creatinine should be checked. The panel has decided not to make recommendations for specific agents for particular procedures as there is considerable variation in Europe and worldwide regarding bacterial pathogens, their susceptibility and availability of antibiotic agents.
3.17.2. Specific procedures and evidence question
An updated literature search from February 2017 (cut-off of last update) to June 2021 identified RCTs, systematic reviews and meta-analyses that investigated the benefits and harms of using antibiotic prophylaxis prior to specific urological procedures. The available evidence enabled the panel to make recommendations concerning urodynamics, cystoscopy, stone procedures (extracorporeal shockwave lithotripsy [ESWL], ureteroscopy and percutaneous nephrolithotomy [PCNL]), transurethral resection of the prostate (TURP) and transurethral resection of the bladder (TURB). For nephrectomy and prostatectomy the scientific evidence was too weak to allow the panel to make recommendations either for or against antibiotic prophylaxis. The general evidence question was: Does antibiotic prophylaxis reduce the rate of post-operative symptomatic UTI in patients undergoing each named procedure?
3.17.2.1. Urodynamics
The literature search identified one systematic review for antibiotic prophylaxis in women only [581]. This included three RCTs (n=325 patients) with the authors reporting that prophylactic antibiotics reduced the risk of bacteriuria but not clinical UTI after urodynamics [581]. A previous Cochrane review identified nine RCTs enrolling 973 patients with overall low quality and high or unclear risks of bias [582]. The outcome of clinical UTI was reported in four trials with no benefit found for antibiotic prophylaxis vs. placebo [RR (95%CI) 0.73 (0.52-1.03)]. A meta-analysis of nine trials showed that use of antibiotics reduced the rate of post-procedural bacteriuria [RR (95%CI) 0.35 (0.22-0.56)] [582].
3.17.2.2. Cystoscopy
Three systematic reviews and meta-analyses [583-585] and one additional RCT [586] on cystoscopy for stent removal were identified. The first study included seven RCTs with a total of 3,038 participants. The outcome of symptomatic UTI was measured by five trials of moderate overall quality and meta-analysis showed a benefit for using antibiotic prophylaxis [RR (95%CI) 0.53 (0.31 – 0.90)]; ARR 1.3% (from 2.8% to 1.5%) with a NNT of 74 [584]. This benefit was not seen if only the two trials with low risk of bias were used in the meta-analysis. The second study included seven RCTs with 5,107 participants. Six trials were included in meta-analysis of the outcome of symptomatic bacteriuria which found benefit for use of antibiotic prophylaxis [RR (95%CI) 0.34 (0.27 – 0.47)]; ARR 3.4% (from 6% to 2.6%) with NNT of 28 [583]. The third study included twenty RCTs and two quasi-RCTs with a total of 7,711 participants. The outcome of symptomatic UTI was measured by eleven RCTs of low overall quality and meta-analysis showed a possible benefit for using antibiotic prophylaxis [RR (95% CI) 0.49 (0.28 – 0.86)] [585]. For systemic UTI, antibiotic prophylaxis showed no effect compared with placebo or no treatment in five RCTs [RR (95% CI) 1.12 (0.38 - 3.32)]. However, prophylactic antibiotics may increase bacterial resistance [(RR (95% CI) 1.73 (1.04 – 2.87)].
Given the low absolute risk of post-procedural UTI in well-resourced countries, the high number of procedures being performed, and the high risk of contributing to increasing antimicrobial resistance, the panel consensus was to strongly recommend not to use antibiotic prophylaxis in patients undergoing urethrocystoscopy (flexible or rigid).
3.17.2.3. Interventions for urinary stone treatment
3.17.2.3.1. Extracorporeal shockwave lithotripsy
For patients without bacteriuria undergoing ESWL, two systematic reviews and meta-analyses were identified with latest search dates of November 2011 and October 2012, respectively [587,588] and two further trials [589]. One review included nine RCTs with a total of 1,364 patients and found no evidence of benefit in terms of reducing the rate of post-procedural fever or bacteriuria [587]. One study included eight RCTs with a total of 940 participants and found no evidence of benefit for antibiotic prophylaxis to reduce rate of fever or trial-defined infection [588]. An RCT with 274 patients and severe risk of bias found no reduction in fever at up to one week post-procedure using a single dose of levofloxacin 500 mg and no difference in the rate of bacteriuria [589]. Another RCT (n = 600) again with severe risk of bias found no difference in UTI and positive urine culture rates at two weeks post-procedure using 200 mg ofloxacin post-operatively for three-days vs. placebo [590].
For patients with bacteriuria or deemed at high risk of complications, one RCT comparing the use of ofloxacin or trimethoprim-sulphamethoxazole for three days prior and four days subsequent to ESWL in 56 patients with ureteric stents was identified [591]. They found no difference in rate of clinical UTI at seven days (no events) and no difference in post-ESWL bacteriuria.
3.17.2.3.2. Ureteroscopy
One updated systematic review and meta-analysis with last search date of June 2017 was identified and included eleven RCTs with 4,591 patients [592]. The meta-analysis found that post-operative pyuria and bacteriuria rates were significantly lower in patients who received pre-operative antibiotic prophylaxis pyuria (OR: 0.42, 95% CI: 0.25–0.69 and OR: 0.25, 95% CI: 0.11–0.58, respectively). Five studies assessed post-operative febrile UTI (fUTI) and found no difference in the rate of fUTIs between patients who did or did not receive antibiotic prophylaxis (OR: 0.82, 95% CI: 0.40– 1.67; p = 0.59). However, a significantly higher risk of post-operative fever in the pre-operative antibiotic prophylaxis group (OR: 1.75, 95% CI: 1.22–2.50; p = 0.002) was reported. A subgroup analysis on the type of pre-operative antibiotic prophylaxis found no difference between a single dose of oral vs. intravenous antibiotics [592].
An RCT comparing different ciprofloxacin-based antibiotic prophylaxis regimens on the incidence of SIRS after URS found there was no difference in the incidences of SIRS between the regimens including the zero-dose regime [593]. However, there was a greater risk of SIRS in patients who did not receive antibiotic prophylaxis when the stone size was > 200 mm2 [593]. Another RCT comparing the use of two oral doses of 3g Fosfomycin tromethamine before surgery to standard of care did not find any difference in the incidence of infections, bacteriuria or fever [594].
Panel discussion considered that despite low quality evidence suggesting no benefit in reducing risk of clinical UTI, clinicians and patients would prefer to use prophylaxis to prevent kidney infection or sepsis. Ideally this should be examined in a robustly designed clinical study.
3.17.2.3.3. Percutaneous neprolithotomy (PNL)
The largest systematic review and meta-analysis performed, latest search date April 2019, included 1,549 patients in thirteen comparative studies on antibiotic prophylaxis strategies for PNL [595]. Compared with a single dose before surgery pre-operative antibiotic prophylaxis significantly reduced post-operative sepsis and fever (OR 0.31, 95% CI: 0.20-0.50 and OR 0.26, 95% CI: 0.14-0.48, respectively) [595]. Similarly, the rate of positive pelvic urine and positive stones culture were reduced when pre-operative prophylaxis was given. There was no difference in sepsis rates between patients receiving or not receiving post-operative prophylaxis; however, patients who received post-operative antibiotic prophylaxis had more fever [595].
Four RCTs with overall low risk of bias comparing different antibiotic regimes in PNL were identified [596-599]. A study compared the rate of SIRS following PNL in 191 patients receiving either a combination of sulbactam/ampicillin or cefuroxime. There was no difference in SIRS or urosepsis rates [596]. Another study investigated single dose ceftriaxone versus ceftriaxone and subsequently an oral third-generation cephalosporin until after nephrostomy catheter withdrawal at mean (SD) of 3 (1) days in 73 participants undergoing PNL. They found no difference in rate of infectious complications between the two antibiotic regimens [597]. Another study compared the administration of 1g ceftriaxone and 1g cefazoline both administered 30 minutes before surgery and continued till nephrostomy removal. They found no difference in terms of SRIS or sepsis between groups [599]. A study compared ciprofloxacin 200 mg IV vs. 2 mg cefotaxime 30 minutes before and twelve hours after surgery and found a higher rate of fever in the cefotaxime group [598]. However, these results remain limited by the high risk of bias and the lack of data regarding post-operative infection. These studies give moderate evidence that a single dose of a suitable agent was adequate for prophylaxis against clinical infection after PNL.
3.17.2.4. Transurethral resection of the prostate
A systematic review of 39 RCTs with search date up to 2009 was identified [600]. The update search to February 2017 did not reveal any further relevant studies. Of the 39 RCTs reviewed, six trials involving 1,666 men addressed the risk of septic episodes, seventeen trials reported procedure related fever and 39 investigated bacteriuria. Use of prophylactic antibiotics compared to placebo showed a relative risk reduction (95% CI) for septic episode of 0.51 (0.27-0.96) with ARR of 2% (3.4% - 1.4%) and a NNT of 50. The risk reduction (95% CI) for fever was 0.64 (0.55-0.75) and 0.37 (0.32-0.41) for bacteriuria.
3.17.2.5. Transurethral resection of the bladder
One systematic review which included seven trials with a total of 1,725 participants was identified [601]. Antimicrobial prophylaxis showed no significant effect on post-operative UTIs [OR (95% CI) 1.55 (0.73 - 3.31)] and asymptomatic bacteriuria [OR (95% CI) 0.43 (0.18 – 1.04)] [601]. The review did not attempt sub-group analysis according to presence of risk factors for post-operative infection such as tumour size. Risk factors for development of post-operative UTIs were evaluated only by three of the included studies and most of the parameters were analysed by no more than one study.
An RCT (n = 100) comparing oral fosfomycin 3g (the night before surgery) vs. intravenous cefoxitin 2g (30 min pre- and 24 hrs post-surgery) on post-operative UTIs found that a single oral administration of fosfomycin was non-inferior to intravenous administration of cefoxitin in the prevention of post-TURB UTI, even in patients considered at higher risk [602].
Panel discussion concluded that a weak recommendation to use antibiotic prophylaxis for patients undergoing TURB who had a high risk of suffering post-operative sepsis would be appropriate.
3.17.2.6. Midurethral slings
One systematic review and meta-analysis identified one study assessing the role of pre-operative antibiotics for midurethral sling surgery alone [603]. The study was halted due to low rate of infectious outcomes seen at the first scheduled interim analysis. The study enrolled 29 women in the antibiotic prophylaxis (cefazolin) group and 30 in the placebo group with a total follow-up of six months. No statistically significant difference between the cefazolin and placebo groups, with respect to wound infections [1 (3.3%) and 0 (0%)] or bacteriuria [3 (10%) and 1 (3.5%)] was found [603].
3.17.2.7. Renal tumour ablation
One systematic review publication date 2018 included 6,952 patients across 51 studies [604]. Infectious complications were reported in 74 patients including fever (60.8%), abscess (21.6%) and UTI (8.1%). Prophylactic antibiotic use was reported in 5.4% of patients but it was not possible to study its association to infectious complications due to lack of reporting.
3.17.2.8. Prostate biopsy
3.17.2.8.1. Transperineal prostate biopsy
Due to three new RCT [605-608], comparing the route of biopsy, an updated meta-analysis was performed by the panel [609]. A total of eleven randomised studies including 3,306 patients compared the impact of biopsy route on infectious complications. Infectious complications were significantly higher following transrectal biopsy (64 events among 1,638 men) compared to transperineal biopsy (32 events among 1,668 men), [RR (95% CIs) 1.85 (1. 07 to 3.19)] [605-607,609,610]. In addition, a systematic review including 165 studies with 162,577 patients described sepsis rates of 0.1% and 0.9% for transperineal and transrectal biopsies, respectively [611]. Finally, a population-based study from the UK (n=73,630) showed lower re-admission rates for sepsis in patients who had transperineal versus transrectal biopsies (1.0% vs, 1.4%, respectively) [612].
A systematic review and meta-analysis of eight non-RCTs reported no significant differences between patients receiving or not receiving antibiotic prophylaxis in terms of post-biopsy infection (0.11% vs. 0.31%) and sepsis (0.13% vs. 0.09%), for the transperineal approach [613]. This is in line with another systematic review and meta-analysis of 112 individual patient cohorts which also showed no significant difference in the number of patients experiencing post-transperineal biopsy infection with 1.35% of 29,880 patients receiving antibiotic prophylaxis and 1.22% of 4,772 not receiving antibiotic prophylaxis (p = 0.8) [614]. In addition, two recently published RCTs have reported comparably low post-biopsy infection rates for transperineal biopsy regardless of whether antibiotic prophylaxis was administered or not [615,616].
There is a growing body of evidence to suggest that antibiotic prophylaxis may not be required for transperineal biopsy; however, as all recommendations on prostate biopsy are based on level 1a evidence, the Panel has chosen to wait until a number of ongoing RCTs report their study findings before making a recommendation on this.
3.17.2.8.2. Transrectal prostate biopsy
An updated meta-analysis of eleven RCTs including 2,237 men showed that use of a rectal povidone-iodine preparation before biopsy, in addition to antimicrobial prophylaxis, resulted in a significantly lower rate of infectious complications [RR (95% CIs) 0.47 (0.36 to 0.61) [609,617-619]. Single RCTs showed no evidence of benefit for perineal skin disinfection [620], but reported an advantage for rectal povidone-iodine preparation before biopsy compared to after biopsy [621]. Therefore, routine surgical disinfection of the perineal skin before performing a transperineal biopsy is recommended.
A meta-analysis of four RCTs including 671 men evaluated the use of rectal preparation by enema before transrectal biopsy. No significant advantage was found regarding infectious complications [RR (95% CIs) 0.96 (0.64 to 1.54) [609].
An updated meta-analysis of 29 RCTs with 4,127 patients found no evidence that use of peri-prostatic injection of local anaesthesia resulted in more infectious complications than no injection [RR (95% CIs) 1.08 (0.80 to 1.49)] [609,610,622,623]. An updated meta-analysis of ten RCTs including 2,342 patients found that extended biopsy templates showed comparable infectious complications to standard templates [RR (95% CIs) 0.82 (0.55 to 1.24)] [609,624]. Additional meta-analyses found no difference in infections complications regarding needle guide type (disposable vs. reusable), needle type (coaxial vs. non-coaxial), needle size (large vs. small), and number of injections for peri-prostatic nerve block (standard vs. extended) [609].
A meta-analysis of eleven studies with 1,753 patients showed significantly reduced infections after transrectal prostate biopsy when using antimicrobial prophylaxis as compared to placebo/control [RR (95% CIs) 0.56 (0.40 to 0.77)] [625].
Fluoroquinolones have been traditionally used for antibiotic prophylaxis in this setting; however, overuse and misuse of fluoroquinolones has resulted in an increase in fluoroquinolone resistance. In addition, the European Commission has implemented stringent regulatory conditions regarding the use of fluoroquinolones resulting in the suspension of the indication for peri-operative antibiotic prophylaxis including prostate biopsy [164].
A systematic review and meta-analysis on antibiotic prophylaxis for the prevention of infectious complications following prostate biopsy concluded that in countries where fluoroquinolones are allowed as antibiotic prophylaxis, a minimum of a full one-day administration is recommended [625]. An updated meta-analysis of nine RCTs with 3,096 patients confirmed that targeted therapy (antibiotic guidance based on rectal swab microbiology) in case of fluoroquinolone resistance is associated with reduced infectious complications [RR (95% CIs) 0.52 (0.39 to 0.69)] [625,626]. In addition, an updated meta-analysis of ten RCTs with 2,787 patients comparing augmented prophylaxis (combination of two or more different classes of antibiotics) to standard prophylaxis, showed augmented prophylaxis to be superior [RR (95% CIs) 0.44 (0.32 to 0.59)] [625,627]. In countries where the use of fluoroquinolones is suspended, cephalosporins or aminoglycosides can be used as individual agents with comparable infectious complications based on a meta-analysis of two RCTs [625]. An updated meta-analysis of four RCTs compared fosfomycin trometamol to fluoroquinolones [RR (95% CIs) 0.62 (0.37 to 1.06)] [625,628]. Although initial RCTs suggested fosfomycin trometamol to be superior, the latest Swedish study, which aimed to recruit 3,448 patients, was discontinued after 42 patients due to the unusually high number of hospitalisations in the fosfomycin trometamol group [628]. Therefore, routine general use should be critically assessed due to the relevant infectious complications also reported in non-randomised studies [629]. Of note, the indication of fosfomycin trometamol for prostate biopsy has been withdrawn in Germany as the manufacturers did not submit the necessary pharmacokinetic data in support of this indication. Urologists are advised to check their local guidance in relation to the use of fosfomycin trometamol for prostate biopsy. Another possibility is the use of augmented prophylaxis without fluoroquinolones, although no standard combination has been established to date. Finally, targeted prophylaxis based on rectal swap/stool culture is plausible, but no RCTs are available on non-fluoroquinolones. See figure 4 for prostate biopsy workflow to reduce infections complications.
3.17.3. Summary of evidence and recommendations for peri-procedural antibiotic prophylaxis
| Summary of evidence | LE |
| The outcome of clinical UTI was reported in four out of eleven RCTs with no benefit found for antibiotic prophylaxis vs. placebo in patients following filling and voiding cystometry. | 1b |
| A meta-analysis of five trials of moderate quality showed a benefit for using antibiotic prophylaxis for the reduction of symptomatic UTI in patients undergoing cystoscopy. However, this benefit was not seen if only the two trials with low risk of bias were used in the meta-analysis. | 1a |
| Two meta-analyses found no benefit for antibiotic prophylaxis following ESWL in terms of reducing the rate of post-procedural fever and bacteriuria or trial-defined infection in patients without bacteriuria. | 1a |
| Two meta-analyses found no evidence of benefit for antibiotic prophylaxis prior to ureteroscopy in reducing the rate of clinical UTI; however, the rate of bacteriuria was reduced. | 1a |
| A meta-analysis of five RCTs demonstrated a moderate level of evidence that antibiotic prophylaxis was associated with a statistically significant reduction in the risk of post-procedural UTI following PNL. | 1a |
| Two RCTs concluded that a single dose of a suitable agent was adequate for prophylaxis against clinical infection after PNL. | 1b |
| A systematic review of 39 RCTs concluded that antibiotic prophylaxis reduced the rate of infectious complications in men undergoing TURP. | 1b |
| A systematic review of two RCTs found no benefit for antibiotic prophylaxis in patients undergoing TURB. | 1b |
| A meta-analysis of elevenstudies including 3,306 patients showed significantly reduced infectious complications in patients undergoing transperineal biopsy as compared to transrectal biopsy. | 1a |
| A meta-analysis of eight non-RCTS reported comparable rates of post-biopsy infections in patients undergoing transperineal biopsy irrespective if antibiotic prophylaxis was given or not. | 2 |
| A meta-analysis of eleven RCTs including 2,237 men showed that use of a rectal povidone-iodine preparation before transrectal biopsy, in addition to antimicrobial prophylaxis, resulted in a significantly lower rate of infectious complications. | 1a |
| An updated meta-analysis of nine RCTs with 3,096 patients confirmed that targeted therapy (antibiotic guidance based on rectal swab microbiology) in case of fluoroquinolone resistance is associated with reduced infectious complications. | 1a |
| A meta-analysis of ten RCTs with 2,787 patients comparing augmented prophylaxis (com-bination of two or more different classes of antibiotics) to standard prophylaxis showed augmented prophylaxis to be superior. | 1a |
| Recommendations | Strength rating |
Do not use antibiotic prophylaxis to reduce the rate of symptomatic urinary infection following:
| Strong |
| Use antibiotic prophylaxis to reduce the rate of symptomatic urinary infection following ureteroscopy. | Weak |
| Use single dose antibiotic prophylaxis to reduce the rate of clinical urinary infection following percutaneous nephrolithotomy. | Strong |
| Use antibiotic prophylaxis to reduce infectious complications in men undergoing transurethral resection of the prostate. | Strong |
| Use antibiotic prophylaxis to reduce infectious complications in high-risk patients undergoing transurethral resection of the bladder. | Weak |
| Perform prostate biopsy using the transperineal approach due to the lower risk of infectious complications and better antibiotic stewardship. | Strong |
| Use rectal cleansing with povidone-iodine in men prior to transrectal prostate biopsy. | Strong |
| Do not use fluoroquinolones for prostate biopsy in line with the European Commission final decision on EMEA/H/A-31/1452. | Strong |
For antibiotic prophylaxis in transrectal biopsy*, and from an antimicrobial stewardship perspective, the following options are recommended**:
| Strong |
*Of note, the indication of fosfomycin trometamol for prostate biopsy has been withdrawn in Germany as the manufacturers did not submit the necessary pharmacokinetic data in support of this indication. Urologists are advised to check their local guidance in relation to the use of fosfomycin trometamol for prostate biopsy.
**While most studies have been performed using fluoroquinolones, the applicability of these findings to non-fluoroquinolone antibiotics remains unclear.
Table 16: Suggested regimens for antimicrobial prophylaxis prior to urological procedures
As stated in section 3.14.1.4, the panel has decided not to make recommendations for specific agents for particular procedures, those listed below represent possible choices only. Urologists should choose a specific antimicrobial based on their knowledge of local pathogen prevalence for each type of procedure, their antibiotic susceptibility profiles and virulence.
| Procedure | Prophylaxis recommended | Antimicrobial |
| Urodynamics | No | N/A |
| Cystoscopy | No | |
| Extracorporeal shockwave lithotripsy | No | |
| Ureteroscopy | Yes | Trimethoprim Trimethoprim-sulphamethoxazole Cephalosporin group 2 or 3 Aminopenicillin plus a beta-lactamase inhibitor |
| Percutaneous nephrolithotomy | Yes (single dose) | |
| Transurethral resection of the prostate | Yes | |
| Transurethral resection of the bladder | Yes, in patients who have a high risk of suffering post-operative sepsis. | |
| Transrectal prostate biopsy | Yes | 1. Targeted prophylaxis - based on rectal swab or stool culture. 2. Augmented prophylaxis - two or more different classes of antibiotics*. 2. Alternative antibiotics
|
* Note option 2 is against antibiotic stewardship programmes
** Of note, the indication of fosfomycin trometamol for prostate biopsy has been withdrawn in Germany as the manufacturers did not submit the necessary pharmacokinetic data in support of this indication. Urologists are advised to check their local guidance in relation to the use of fosfomycin trometamol for prostate biopsy.
Figure 4: Prostate biopsy workflow to reduce infectious complications 1. Two systematic reviews including non-RCTs and two RCTs describe comparable rates of post-biopsy infection in patients with and without antibiotic prophylaxis.
1. Two systematic reviews including non-RCTs and two RCTs describe comparable rates of post-biopsy infection in patients with and without antibiotic prophylaxis.
2. Be informed about local antimicrobial resistance.
3. Banned by European Commission due to side effects.
4. Contradicts principles of Antimicrobial Stewardship.
5. Fosfomycin trometamol (4 RCTs), cephalosporins (2 RCTs), aminoglycosides (2 RCTs).
6. Only one RCT comparing targeted and augmented prophylaxis.
7. Originally introduced to use alternative antibiotics in case of fluoroquinolone resistance.
8. Various schemes: fluoroquinolone plus aminoglycoside (4 RCTs); and fluoroquinolone plus cephalosporin (1 RCT).
9. Significantly inferior to targeted and augmented prophylaxis.
High certainty: (⊕⊕⊕⊕)very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: (⊕⊕⊕○)moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: (⊕⊕○○) confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: (⊕○○○) very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. Figure reproduced from Pilatz et al., with permission from Elsevier[630]
* Of note the indication of fosfomycin trometamol for prostate biopsy has been withdrawn in Germany as the manufacturers did not submit the necessary pharmacokinetic data in support of this indication. Urologists are advised to check their local guidance in relation to the use of fosfomycin trometamol for prostate biopsy.