5. DIAGNOSTIC EVALUATION
5.1. Primary diagnosis
5.1.1. Symptoms
Painless visible haematuria is the most common presenting complaint. Other presenting symptoms and clinical signs include nonvisible haematuria, urgency, dysuria, increased frequency, and in more advanced tumours, pelvic pain and symptoms related to urinary tract obstruction.
5.1.2. Physical examination
Physical examination should include rectal and vaginal bimanual palpation. A palpable pelvic mass can be found in patients with locally-advanced tumours. In addition, bimanual examination under anaesthesia should be carried out before and after TUR of the bladder tumour (TURBT) to assess whether there is a palpable mass or if the tumour is fixed to the pelvic wall [89,90]. However, considering the discrepancy between bimanual examination and pT stage after cystectomy (11% clinical overstaging and 31% clinical understaging), bimanual examination findings need to be interpreted with caution [91].
5.1.3. Bladder imaging
Patients with a bladder mass identified by any diagnostic imaging technique should undergo cystoscopy, biopsy and/or resection for histopathological diagnosis and staging.
Due to the high specificity of diagnostic imaging for detecting BC, patients with imaging positive for BC may avoid diagnostic flexible cystoscopy and go directly for TUR [92,93].
5.1.4. Urinary cytology
Examination of voided urine or bladder washings for exfoliated cancer cells has high sensitivity in high-grade tumours and is a useful indicator in cases of high-grade malignancy or CIS. However, positive urinary cytology may originate from an urothelial tumour located anywhere in the urinary tract.
Evaluation of cytology specimens can be hampered by low cellular yield, UTIs, stones or intravesical instillations, but for experienced readers, specificity exceeds 90% [94,95]. However, negative cytology does not exclude a tumour. There is no known urinary marker specific for the diagnosis of invasive BC [96].
A standardised reporting system, the ‘Paris System’ redefining urinary cytology diagnostic categories has been updated in 2022 [97]:
- adequacy of urine specimens (Adequacy);
- negative for high-grade UC (Negative);
- atypical urothelial cells (AUC);
- suspicious for high-grade UC (SHGUC);
- high-grade UC (HGUC).
5.1.5. Cystoscopy
Ultimately, the diagnosis of BC is made by cystoscopy and histological evaluation of resected tissue. An (outpatient) flexible cystoscopy is recommended to obtain a complete image of the bladder. However, in daily practice, if a bladder tumour has been visualised unequivocally by imaging studies such as computed tomography (CT), magnetic resonance imaging (MRI), or ultrasound (US), diagnostic cystoscopy may be omitted and the patient can proceed directly to TURB for resection and histological diagnosis. During the procedure, a thorough inspection of the bladder wall with rigid cystoscopy under anaesthesia is mandatory in order not to miss any tumours.
A careful description of the cystoscopic findings is necessary. This should include documentation of the site, size, number, and appearance (papillary or solid) of the tumours, as well as a description of any mucosal abnormalities [98]. The use of a bladder diagram is recommended.
The use of photodynamic diagnosis (PDD) could be considered in multifocal tumours and in case CIS is suspected. Presence of CIS may lead to a modified treatment plan (see EAU Guidelines on Non-muscle-invasive Bladder Cancer [2]). Photodynamic diagnosis is highly sensitive for the detection of CIS and in experienced hands the rate of false-positive results may be similar to that with regular white-light cystoscopy [85,99].
5.1.6. Transurethral resection of invasive bladder tumours
The goal of TURB is to enable histopathological diagnosis and staging, which requires the inclusion of bladder muscle in the resection specimen.
In case MIBC is suspected, tumours need to be (ideally) resected separately in parts, which include the exophytic part of the tumour, the underlying bladder wall with the detrusor muscle, and the edges of the resection area. The deeper portion of the resection specimen should be sent to the pathologist in a separate clearly labelled container to ensure accurate diagnosis and staging. If RT is being considered and CIS needs to be excluded, PDD can be used [100].
The involvement of the prostatic urethra and ducts in men with bladder tumours has been reported in up to one in three patients [58,101,102]. Under-reporting possibly also means that the exact risk is not known, but it seems to be higher if the tumour is located on the trigone or bladder neck, with concomitant bladder CIS, and in the case of multiple tumours [57,103,104]. Involvement of the prostatic urethra can be determined either at the time of primary TURB or by frozen section during the cystoprostatectomy procedure. A frozen section has a higher negative-predictive value and is more accurate [105-107].
A negative urethral frozen section can reliably identify patients in whom urethrectomy should be avoided. However, a positive pre-operative biopsy seems to have limited utility as these findings are not reliably associated with final margin status [105,108].
Diagnosis of a urethral tumour before cystectomy will result in a urethrectomy which could be a contraindication for an orthotopic diversion. However, an orthotopic diversion should not be denied based on positive pre-operative biopsy findings alone and frozen section should be part of the RC procedure, particularly in male patients [109,110].
5.1.7. Summary of evidence and recommendations for the primary assessment of presumably invasive bladder tumours
| Summary of evidence | LE |
| Cystoscopy is necessary for the diagnosis of bladder cancer. | 1 |
| Urinary cytology has high sensitivity in high-grade tumours including carcinoma in situ. | 2b |
| In men, prostatic urethral biopsy includes resection from the bladder neck to the verumontanum (between the 5 and 7 o’clock position) using a resection loop. In case any abnormal-looking areas in the prostatic urethra are present at this time, these need to be biopsied as well. | 2b |
| Recommendations | Strength rating |
| Describe all macroscopic features of the tumour (site, size, number and appearance) and mucosal abnormalities during cystoscopy. Use a bladder diagram. | Strong |
| Take a biopsy of the prostatic urethra in cases of bladder neck tumour, when bladder carcinoma in situ is present or suspected, when there is positive cytology without evidence of tumour in the bladder, or when abnormalities of the prostatic urethra are visible. | Strong |
| In men with a negative prostatic urethral biopsy undergoing subsequent orthotopic neobladder construction, an intra-operative frozen section can be omitted. | Strong |
| In men with a prior positive transurethral prostatic biopsy, subsequent orthotopic neobladder construction should not be denied a priori, unless an intra-operative frozen section of the distal urethral stump reveals malignancy at the level of urethral dissection. | Strong |
| In women undergoing subsequent orthotopic neobladder construction, obtain procedural information (including histological evaluation) of the bladder neck and urethral margin, either prior to, or at the time of cystectomy. | Strong |
| In the pathology report, specify the grade, depth of tumour invasion, and whether the lamina propria and muscle tissue are present in the specimen. | Strong |
For general information on the assessment of bladder tumours, see EAU Guidelines on Non-muscle-invasive Bladder Cancer [2]
5.2. Imaging for staging of MIBC
In clinical practice, tumour stage and histopathological grade are used to guide treatment and determine prognosis [111-113]. Imaging is essential for local- and distant-staging of BC.
The goal of imaging patients with BC is to:
- Detect bladder tumours;
- Differentiate T1 from T2 tumours as their treatment will differ;
- Determine presence of any obstruction to the upper UT;
- Evaluate the extent of locally-advanced tumour stage or tumour spread to LNs;
- Assess synchronous tumour in the upper UT or other distant organs (e.g., liver, lungs, bones, peritoneum, pleura, and adrenal glands).
Table 5.1: The role of imaging in treatment planning
| Goal | Imaging modality |
| Differentiate T1 from T2 tumours | MRI using the Vesical Imaging Reporting and Data System (VI-RADS) score |
| Evaluate locally-advanced stage or spread to LNs | CT scan and MRI for abdominal- and pelvic LNs or PET/CT scan |
| Assess UUT or other distant organs | CT urography for evaluating the UUT and PET/CT to detect distant organ metastasis |
5.2.1. Detection
Imaging modalities used to detect bladder tumours are: US, CT and MRI-scan. Bladder tumours are often detected as part of the haematuria work-up (including cystoscopy) or as an incidental finding on imaging.
Ultrasound can visualise intraluminal masses in the bladder and additional signs such as hydronephrosis, but cannot rule out all possible causes of haematuria. According to the results of the DETECT I trial, CT urogram can be safely replaced by renal and bladder US in patients who have nonvisible haematuria [114].
5.2.2. Local staging of the bladder and upper tract
5.2.2.1. Magnetic resonance imaging for local staging of MIBC
Differentiation between NMIBC and MIBC is crucial for BC treatment. Magnetic resonance imaging has superior soft tissue contrast resolution compared with CT and can evaluate post-biopsy reaction as enhancement of the tumour occurs earlier than that of the normal bladder wall due to neovascularisation [115,116]. However, MR is not yet ready for standard patient care [117].
Multiparametric (mp)MRI using the Vesical Imaging – Reporting and Data System (VI-RADS) scoring system has been used to differentiate between T1 vs. T2 bladder tumours with a high diagnostic accuracy [118]. The VI-RADS offers a standardised approach to both acquisition and reporting of mpMRI for BC; however, the best practice of using mpMRI in this setting and the exact cut-off levels for VI-RADS scoring still need to be determined [116]. To date, the VI-RADS score has been validated by several research groups, showing good diagnostic performance in detecting MIBC [119,120]. In addition, a high diagnostic performance for the detection of muscle invasion of UC subtypes was found [121].
VI-RADS assessment scoring proved to be an independent predictor of muscle-invasiveness, which might facilitate a shift toward a more aggressive approach for selection of patients at high risk of MIBC, according to a novel proposed predictive pathway [122].
A meta-analysis found that the pooled sensitivity and specificity of mpMRI with VI-RADS acquisition and scoring for predicting MIBC were 83% and 90%, respectively [123]. The diagnostic performance of using VI-RADS scoring is similar to the diagnostic performance of a conventional bladder MRI in determining MIBC based on a previous meta-analysis of 24 studies [123]. The analysis found substantial inter-reader agreement, with kappa (κ) values ranging from 0.81 to 0.92 [123]. The potential role of mpMRI as first-line test for local staging of BC rather than TURB has been demonstrated in a recent clinical trial [124].
A modified Delphi methodology was developed by a panel of highly experienced, internationally recognised radiologists, urologists, oncologists, radiation oncologists and a representative from a patient advocacy group, to provide consensus-based recommendations for urinary bladder MRI to help formulate international guidelines, particularly for pre-operative cancer staging and the assessment of the response to systemic therapy. Among several statements that reached agreement, experts recommend acquiring and interpreting MR images according to VI-RADS recommendations and if MRI is performed for primary staging purposes, it should be done before TURBT [125] .
Considering the link established between the use of gadolinium-based contrast agents and nephrogenic systemic fibrosis (NSF) in patients with impaired renal function, contrast medium should be managed according to the European Society of Urogenital Radiology (ESUR) Guidelines [126]. Interest is growing in the role of non-contrast MRI for the assessment of MIBC using VI-RADS with studies demonstrating how non-contrast-enhanced VI-RADS scoring achieved similar predictive accuracy for diagnosis of MIBC to that of conventional VI-RADS; however, further additional evidence is required before any recommendations can be made [127].
5.2.2.2. CT imaging for local staging of MIBC
General advantages of CT imaging include high spatial resolution, shorter acquisition time, wider coverage in a single breath hold, and lower susceptibility to variable patient factors. Computed tomography is unreliable in differentiating between stages Ta to T3a tumours, but it is useful for detecting invasion into the perivesical fat (T3b) and adjacent organs. The accuracy of CT in determining extravesical tumour extension increases with more advanced disease [128].
Both CT and MRI may be used for assessment of local invasion by T3b disease, or higher, but they are unable to accurately diagnose microscopic invasion of perivesical fat (T2 vs. T3a) [129]. Contrast-enhanced CT using iodinated contrast media can be considered as an alternative to MRI when MRI is contraindicated or not available [126].
5.2.2.3. Computed tomography urography for local staging of the upper tract
For local staging of the UUT, computed tomography urography (CTU) has the highest diagnostic accuracy of the available imaging techniques. The sensitivity of CTU for upper urinary tract urothelial carcinoma (UTUC) is 0.67–1.0 and specificity is 0.93–0.99 [130].
Rapid acquisition of thin sections allows high-resolution isotropic images that can be viewed in multiple planes to assist with diagnosis without loss of resolution. Epithelial ‘flat lesions’ without mass effect or urothelial thickening are generally not visible with CT. The secondary sign of hydronephrosis is associated with advanced disease and poor oncological outcome [131]. The presence of enlarged LNs is highly predictive of metastases in UTUC [132].
5.2.2.4. Magnetic resonance urography for local staging of the upper tract
Magnetic resonance urography is indicated in patients who cannot undergo CTU, usually when radiation or iodinated contrast media are contraindicated [133]. The sensitivity of MR-urography is 0.75 after contrast injection for tumours < 2 cm [133]. The use of MR-urography with gadolinium-based contrast media should be limited in patients with severe renal impairment (< 30 mL/min creatinine clearance), due to the risk of NSF. Computed tomography urography is generally preferred to MR-urography for diagnosing and staging UTUC.
5.2.3. Distant staging of lymph nodes and other sites
5.2.3.1. Imaging of lymph nodes in MIBC
Assessment of LN metastases based on size alone is limited; both CT and MRI are unable to identify metastases in normal-sized or minimally-enlarged nodes. The sensitivity of these modalities for detection of LN metastases is low (48–87%). Specificity is also low because nodal enlargement may be due to benign disease. Overall, CT and MRI show similar results in the detection of LN metastases in a variety of primary pelvic tumours [133-135]. Pelvic nodes > 8 mm and abdominal nodes > 10 mm in maximum short-axis diameter, detected by CT or MRI, should be regarded as pathologically enlarged [136]. In a recent paper including 1,104 patients, conventional cross-sectional imaging showed slight concordance (64.9%) between cN and pN stages (sensitivity: 30%; specificity: 84%) [137].
18F-fluorodeoxy glucose-Positron emission tomography (FDG-PET) combined with CT is increasingly being used in clinical practice but its exact role needs to be further evaluated [138,139]. According to a systematic review and meta-analysis including 785 patients, FDG-PET/CT showed a low sensitivity and high specificity for the detection of metastatic LNs in patients with newly diagnosed BC [140]. However, most studies comparing FDG-PET/CT with CT for LN assessment reported higher sensitivity, with comparable specificity [141]. In a comparative analysis PET/CT demonstrated superior diagnosic preformance over contract-enhanced CT; however, up to 20% of occult (micro-) metastases were still missed on final pathology [142].
Positron emission tomography/computed tomography can also provide additional information to guide local treatment in case of the presence of pelvic nodal metastases [143]. A study of 2,731 patients with MIBC showed that pretreatment staging with FDG-PET/CT led to clinical nodal upstaging in approximately one-fifth of cases, impacting treatment decisions [144]. However, both are retrospectieve studies. Additionally, determining the definitive diagnostic accuracy is hampered due to variations in evaluation methods and the increasing use of neoadjuvant therapy.
In addition, the role of PET/CT in evaluating LN involvement in patients receiving neoadjuvant pembrolizumab has been investigated in a clinical trial. The performance of PET/CT did not justify its routine use in cN0 MIBC patients, but proved useful in optimising the selection of MIBC patients suited for neoadjuvant immunotherapy (IO) strategies [145].
5.2.3.2. Distant metastases
Before any curative treatment, it is essential to evaluate the presence of distant metastases. Computed tomography and MRI are the diagnostic techniques of choice to detect e.g., lung [146] and liver metastases [147], respectively.
Evidence for the role of FDG-PET/CT for staging distant metastases of MIBC is still limited. In a recent series of 711 patients, FDG-PET/CT has been shown to provide important staging information through the detection of distant metastases, which may impact the clinical management of MIBC patients [143].
Bone and brain metastases are rare at the time of presentation of invasive BC. In a recent retrospective, large sample, study bone scan has been shown to have an impact on patients’ intended management in only 19 out of 1,148 (1.7%) patients; therefore, it should not be routinely used [148]. Whole- body MRI is more sensitive and specific for diagnosing bone metastases than bone scintigraphy [149]. Also, additional brain imaging is not routinely indicated unless the patient has specific symptoms or signs to suggest brain metastases.
5.2.4. Response to therapy
Pre-operative MRI conducted in various clinical settings may provide useful information regarding treatment response. In the neoadjuvant setting, the first study evaluating the performance of MRI in assessing therapeutic response to chemotherapy showed superiority of DWI over T2-weighted and dynamic contrast-enhanced (DCE)-MRI [150]. The high specificity of DWI indicates its usefulness in accurate predicting a complete histopathological response, allowing for better patient selection for bladder-sparing protocols [151]. Dynamic contrast-enhanced MR imaging may also be useful for predicting a patient’s response to chemotherapy. In addition, quantitative DWI/MRI analysis has shown to provide an accurate and non-invasive assessment of bladder RT response. However, multi-centre validation is required before prospective testing to inform MIBC follow-up schedules and decision making [152].
In the previously cited consensus-based recommendations, experts agreed upon the performance on MRI to assess response to systemic therapy to select patients for radical treatment, for surveillance, and for bladder-sparing surgery [125].
A meta-analysis investigated the predictive role of 18F-FDG PET/CT for assessment of tumour response to neoadjuvant chemotherapy in a total of 278 patients, showed a pooled sensitivity of 0.84 (95% CI: 0.72–0.91), and specificity of 0.75 (95% CI: 0.59–0.86). Among the five studies, only three used both cR and pCR as a reference standard [153].
The performance of PET/CT in evaluating LN involvement in patients receiving neoadjuvant pembrolizumab did not justify its routine use in cN0 MIBC patients [145].
5.2.5. Future perspectives
Potential future application of the VI-RADS score may include prediction of response to treatment as well as peri-operative outcomes using its modified version: the NAC VI-RADS (nacVI-RADS); however, prospective evidence is warranted [154].
VI-RADS and nacVI-RADS have been proven to accurately predict pre- and post-pembrolizumab response in MIBC, being strongly associated with pathological downstaging and survival [117].
Future trends might include image analysis radiomic-based techniques in predicting MIBC. A meta- analysis (n = 860) provided summary estimates for sensitivity and specificity in predicting MIBC of 82% (95% CI: 77–86%) and 81% (95% CI: 76–85%), respectively [155].
Alternative molecular imaging tracers are being studied such as 64CuCl2, [68Ga]Ga-FAPI-46 and 68Ga-FAP-2286 and preliminary investigations of these agents have demonstrated promising results in nodal staging and restaging in MIBC [156,157].
Positron emission tomography/computed tomography combining the benefits of MRI with functional imaging could be envisioned for the detection of metastatic BC lesions not seen on CT in patients who cannot receive intravenous iodine contrast, and may lead to improved treatment planning and monitoring for BC [158].
Among the novel approaches and radiotracers, in a pilot study, Rietbergen et al., showed that the sentinel node (SN) biopsy in bladder cancer using the hybrid tracer ICG- 99mTc-nanocolloid is feasible, and in patients with a successful pre-operative SN mapping using lymphoscintigraphy and SPECT/CT, the intra-operative SN guidance and detection are effective, even outside the extended pelvic lymph node dissection (ePLND) area [159].
5.2.6. Summary of evidence and guidelines for staging in muscle-invasive bladder cancer
| Summary of evidence | LE |
| Imaging as part of staging in MIBC provides information about prognosis and assists in selection of the most appropriate treatment. | 2b |
| The diagnosis of upper tract UC depends on CT urography and, if needed, ureteroscopy. | 2b |
| In local staging, MRI is superior to CT in terms of differentiating T1 from T2 disease. | 2b |
| MRI is accurate for the assessment of tumour response to systemic therapy. | 3 |
| Bone scintigraphy has limited value in the staging of invasive BC. | 3 |
| FDG-PET/CT can provide additional information to guide treatment. | 2b |
| Recommendations | Strength rating |
| If an MRI is performed for local staging of bladder cancer it should be done before TURBT. | Strong |
| In patients with confirmed muscle-invasive bladder cancer, use computed tomography (CT) of the chest, abdomen and pelvis for staging, including some form of CT urography with designated phases for optimal urothelial evaluation. | Strong |
| Use CT urography, unless it is contraindicated for reasons related to contrast adminstration or radiation dose; in that case use MRI. | Strong |
| Offer MRI to assess the response to systemic therapy, which aids in the selection of patients for radical treatment, surveillance, and bladder-sparing surgery. | Weak |
5.3. Muscle-invasive and metastatic bladder cancer and health status
Complications from RC may be directly related to pre-existing comorbidity as well as the surgical procedure, bowel anastomosis, or urinary diversion. A significant body of literature has evaluated the usefulness of age as a prognostic factor for RC, although chronological age is less important than frailty [160-162]. Frailty is a syndrome of reduced ability to respond to stressors. Patients with frailty have a higher risk of mortality and negative side effects of cancer treatment [163]. Controversy remains regarding age, RC and the type of urinary diversion. Radical cystectomy is associated with the greatest risk reduction in disease-related and non- disease-related death in patients aged < 80 years [164].
The largest retrospective study on RC in septuagenarians and octogenarians based on data from the National Surgical Quality Improvement Program database (n = 1,710) showed no significant difference for wound, cardiac, or pulmonary complications. However, the risk of mortality in octogenarians compared to septuagenarians is higher (4.3% vs. 2.3%) [165]. Although some octogenarians successfully underwent a neobladder procedure, most patients were treated with an ileal conduit diversion. It is important to evaluate functioning and quality of life (QoL) of older patients using a standardised geriatric assessment, as well as carrying out a standard medical evaluation [166].
Sarcopenia has been shown to be an independent predictor for OS and CSS in a large multi-centre study with patients undergoing RC for BC [167]. In order to predict CSM after RC in patients receiving NAC, sarcopenia should be assessed after completing chemotherapy [168]. Other risk factors for morbidity include prior abdominal surgery, extravesical disease, and prior RT [169]. Female gender, an increased BMI and lower pre-operative albumin levels are associated with a higher rate of parastomal hernias [170]. Low pre-operative serum albumin is also associated with impaired wound healing, gastrointestinal (GI) complications and a decrease of recurrence-free and OS after RC [171,172]. Therefore, it could be used as a prognostic biomarker for patients undergoing RC.
Metformin has been suggested as having possibly anticancer activity in bladder cancer by inhibiting tumour growth as well as being synergistic with Cisplatin. A systematic review and meta-analysis of 4,006 patients suggests that Metformin use was associated with lower cancer specific and overall mortality in patients with MIBC [173].
5.3.1. Evaluation of comorbidity, frailty and cognition
Evaluation of comorbidity provides a better indicator of life expectancy in MIBC than patient age [174]. Evaluation of comorbidity helps to identify factors likely to interfere with, or have an impact on, treatment and the evolution and prognosis of MIBC [175].
The value of assessing overall health before recommending and proceeding with surgery was emphasised in another study which demonstrated an association between comorbidity and adverse pathological and survival outcomes following RC [176]. Similar results were found for the impact of comorbidity on cancer-specific and other-cause mortality in a population-based competing risk analysis of > 11,260 patients from the Surveillance, Epidemiology, and End Results (SEER) registries. Age carried the highest risk for other-cause mortality but not for increased cancer-specific death, while the stage of locally-advanced tumour was the strongest predictor for decreased CSS [177].
Stratifying older patients according to frailty using a multidisciplinary approach will help select patients most likely to benefit from radical surgery and to optimise treatment outcomes [178]. There are many different screening tools available for frailty and local approaches can be used. Examples include the G8 and the Clinical Frailty Scale (See Table 5.2 and Figure 5.1 below).
Cognitive impairment can be screened for by using a tool such as the mini-COG
(https://mini-cog.com/), which consists of three-word recall and a clock-drawing test, and can be completed within 5 minutes. A score of ≤ 3/5 indicates the need to refer the patient for full cognitive assessment. Patients with any form of cognitive impairment (e.g., Alzheimer’s or vascular dementia) may need a capacity assessment of their ability to make an informed decision, which is an important factor in health status assessment. Cognitive impairment also predicts risk of delirium, which is important for patients undergoing surgery [179].
Table 5.2: G8 screening tool (adapted from [180])
| Items | Possible responses (score) | |
| A | Has food intake declined over the past three months due to loss of appetite, digestive problems, chewing, or swallowing difficulties? | 0 = severe decrease in food intake |
| 1 = moderate decrease in food intake | ||
| 2 = no decrease in food intake | ||
| B | Weight loss during the last three months? | 0 = weight loss > 3 kg |
| 1 = does not know | ||
| 2 = weight loss between 1 and 3 kg | ||
| 3 = no weight loss | ||
| C | Mobility? | 0 = bed or chair bound |
| 1 = able to get out of bed/chair but does not go out | ||
| 2 = goes out | ||
| D | Neuropsychological problems? | 0 = severe dementia or depression |
| 1 = mild dementia | ||
| 2 = no psychological problems | ||
| E | BMI? (weight in kg)/(height in m2) | 0 = BMI < 19 |
| 1 = BMI 19 to < 21 | ||
| 2 = BMI 21 to < 23 | ||
| 3 = BMI ≥ 23 | ||
| F | Takes more than three prescription drugs per day? | 0 = yes |
| 1 = no | ||
| G | In comparison with other people of the same age, how does the patient consider his/her health status? | 0.0 = not as good |
| 0.5 = does not know | ||
| 1.0 = as good | ||
| 2.0 = better | ||
| H | Age | 0 = > 85 |
| 1 = 80–85 | ||
| 2 = < 80 | ||
| Total score | 0–17 |
Figure 5.1: Clinical Frailty Scale©, Version 2.0* [181] 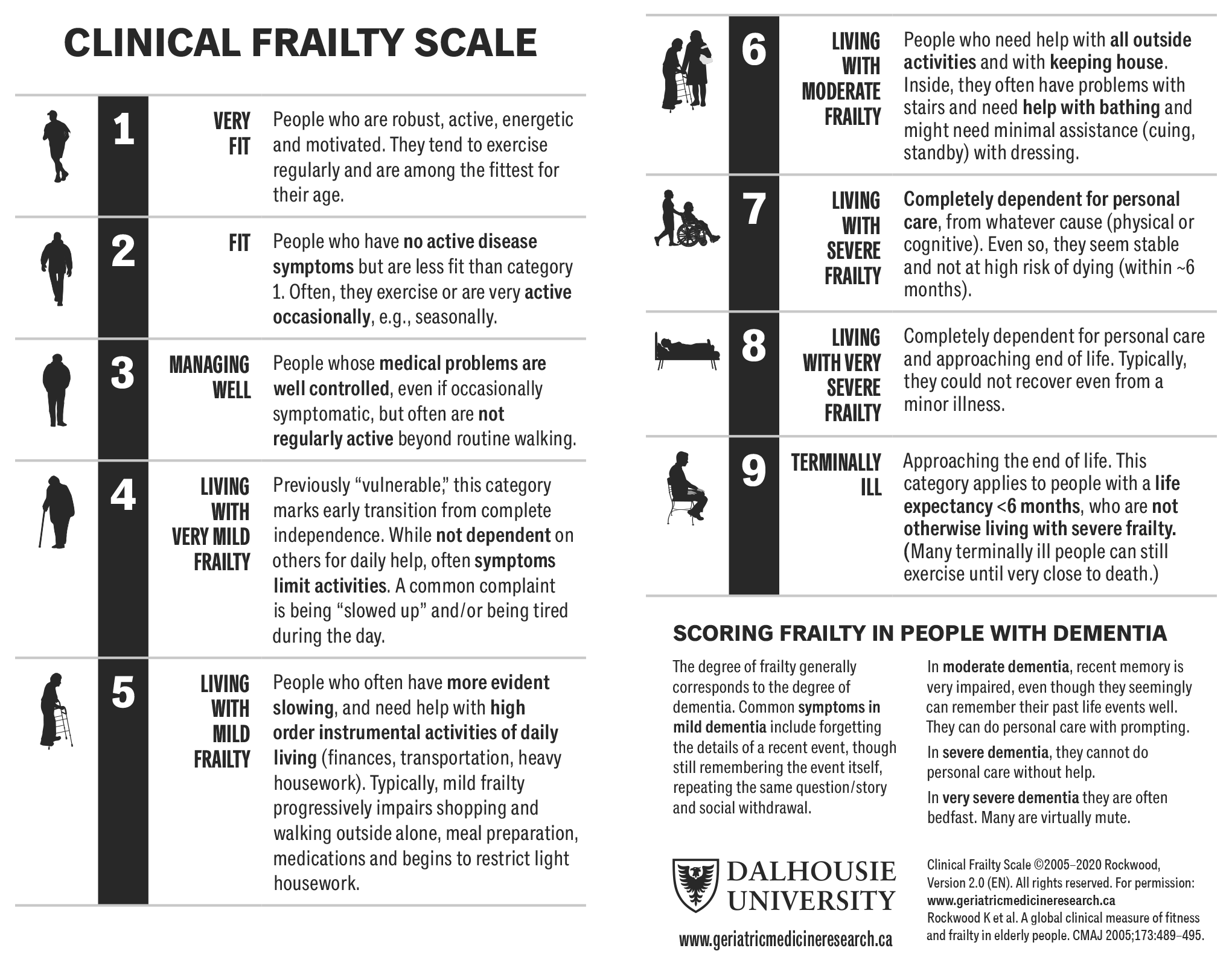 *Permission to reproduce the Clinical Frailty Scale© has been granted by the copyright holder.
*Permission to reproduce the Clinical Frailty Scale© has been granted by the copyright holder.
5.3.2. Comorbidity scales, anaesthetic risk classification and geriatric assessment
A range of comorbidity scales has been developed [182], seven of which have been validated [183-189]. The Charlson Comorbidity Index (CCI) ranges from 0 to 30 according to the importance of comorbidity described at four levels and is calculated by healthcare practitioners based on patients’ medical records. The score has been widely studied in patients with BC and found to be an independent prognostic factor for peri-operative mortality [190,191], overall mortality [192], and CSM [164,193-195]. Only the age-adjusted version of the CCI was correlated with both cancer-specific and other-cause mortality [196]. The age-adjusted CCI (Table 5.3) is the most widely used comorbidity index in cancer for estimating long-term survival and is easily calculated [197].
Health assessment of oncology patients must be supplemented by measuring their activity level. Extermann et al., have shown that there is no correlation between morbidity and competitive activity level [198]. The Eastern Cooperative Oncology Group (ECOG) performance status (PS) scores and Karnofsky index have been validated to measure patient activity [199]. Performance score is correlated with patient OS after RC [194] and palliative chemotherapy [200-202].
Patients who have screened positive for frailty or cognitive impairment benefit from an assessment by a geriatrician. This allows identification of geriatric syndromes and any scope for optimisation. The most complete protocol is the Comprehensive Geriatric Assessment (CGA) [203] which is useful in the care of cancer patients [204]. In BC, the CGA has been used to adapt gemcitabine chemotherapy in previously untreated older patients with advanced BC [205].
Table 5.3: Calculation of the Charlson Comorbidity Index
| Number of points | Conditions |
| 1 | 50–60 years |
| Myocardial infarction | |
| Heart failure | |
| Peripheral vascular insufficiency | |
| Cerebrovascular disease | |
| Dementia | |
| Chronic lung disease | |
| Connective tissue disease | |
| Ulcer disease | |
| Mild liver disease | |
| Diabetes | |
| 2 | 61–70 years |
| Hemiplegia | |
| Moderate to severe kidney disease | |
| Diabetes with organ damage | |
| Tumours of all origins | |
| 3 | 71–80 years |
| Moderate to severe liver disease | |
| 4 | 81–90 years |
| 5 | > 90 years |
| 6 | Metastatic solid tumours |
| AIDS |
Interpretation:
1. Calculate Charlson Comorbidity Score or Index = i
- Add comorbidity score to age score
- Total denoted as ‘i’ in the Charlson Probability calculation (see below).
i = sum of comorbidity score to age score
2. Calculate Charlson Probability (10-year mortality = Y)
- Calculate Y = 10(i x 0.9)
- Calculate Z = 0.983Y (where Z is the 10-year survival)
5.3.3. Summary of evidence and guidelines for comorbidity scales
| Summary of evidence | LE |
| Chronological age is of limited relevance. | 3 |
| It is important to screen for frailty and cognitive impairment and provide a Comprehensive Geriatric Assessment (CGA) where optimisation is needed. | 3 |
| Recommendations | Strength rating |
| Base the decision on bladder-sparing treatment or radical cystectomy in older/frail patients with invasive bladder cancer on tumour stage and frailty. | Strong |
| Assess comorbidity by a validated score, such as the Charlson Comorbidity Index. The American Society of Anesthesiologists score should not be used in this setting (see section 5.3.2). | Strong |