3. EPIDEMIOLOGY AETIOLOGY AND PATHOLOGY
3.1. Definition of penile cancer
More than 95% of penile cancers are SCCs. There are several recognised subtypes of penile SCC with different clinical features and natural history (see Table 3.1). Penile SCC usually arises from the epithelium of the inner prepuce or the glans.
Table 3.1: Histological subtypes of penile carcinomas according to the 2020 WHO Classification [8,9], frequency and outcomes (Modified from [10])
Subtype | Frequency (% of cases) | Mortality (%) | Other features |
HPV-independent SCC | |||
Usual | 45 – 75 | 20 – 38 | Diagnosis of exclusion. Various degrees of differentiation |
Pseudohyperplastic* | < 1 | 0 | Well-differentiated, superficially spreading simulating pseudoepitheliomatous hyperplasia |
Pseudoglandular* | < 1 | 30 | Poorly-differentiated carcinoma with acantholytic pseudolumina simulating glands |
Verrucous | 3 – 8 | 0 | Extremely well-differentiated, broad-based, and pushing tumour front. No metastasis reported |
Cuniculatum | < 1 | 0 | Endophytic labyrinthine growth pattern with broad-based pushing margins. |
Papillary | 2 – 15 | 0 – 6 | Papillae covered by well- to moderately differentiated cells without koilocytes |
Sarcomatoid | 1 -7 | 45 – 90 | Biphasic epithelial and spindle cell neoplasia. Most aggressive and worse prognosis. |
Mixed | 10 – 19 | 3 – 7 | Two or more subtypes in the same specimen. Prognosis is related to the subtypes involved. |
HPV-associated | |||
Basaloid | 4 – 10 | 21 – 67 | Uniform basaloid cells in nests or sheets, with comedonecrosis or abrupt keratinisation. |
Warty | 5 – 10 | 0 – 10 | Condylomatous papillae with central fibrovascular cores and koilocytes. |
Clear cell | < 1 | 20 – 30 | Nests or sheets of cells with ample, clear cytoplasm with central of geographical necrosis. |
Lymphoepithelioma-like | < 1 | Unknown | Poorly differentiated cells intermixed with dense lymphoplasmacytic and eosinophilic infiltrate. |
Mixed | 4 - 10 | 30 - 50 | Mainly Warty-basaloid carcinoma according to the WHO 2022. |
Others | |||
SCC NOS (not-otherwise specified) | Unknown | Unknown | Keratinizing carcinoma. This must be used only when evaluation of p16 is not available. |
Adenosquamous | 1 – 2 | 0–14 | Squamous tumour nests intermixed with a minor mucinous glandular component. |
Mucoepidermoid | Unknown | Unknown | Clear separation between adenosquamous and mucoepidermoid is not provided in the WHO classification. Salivary glands criteria can be applied but there is no consensus. |
HPV = human papillomavirus; SCC = squamous cell carcinoma; WHO = World Health Organization.
* WHO 2022 classification consider these subtypes part of usual SCC.
** This is considered a variant of the cuniculatum carcinoma.
3.2. Epidemiology
Penile cancer incidence varies across the world (Fig. 3.1). In industrialised countries, penile cancer is uncommon, with an overall incidence of around 0.94/100,000 males in Europe and 0.5 in the USA [11,12]. In contrast, in South America, Southeast Asia and parts of Africa, the incidence is much higher and can account for 1–2% of malignant disease in men [12]. The annual age-adjusted incidence is 0.7–3.0 in India, 8.3 in Brazil (per 100,000, respectively) and is higher in parts of Africa such as Uganda [12,13].
In Europe, there is considerable variation across countries. Data from Norway showed an increase in the age-standardised incidence rates in 5-year periods from 2001-2015 compared to the previous periods (0.65/100,000 in 1956–60 vs. 0.91/100,000, in 2011-2015) with an Estimated Annual Percent Change of +0.80% [14]. In the United Kingdom, the age-standardised incidence rate increased 28% between 1993 and 2018. This trend was seen in age groups from 50–79 years old. Incidence rates remained unchanged for both age extremes (< 50 and > 79 years) [15]. Based on 16 cancer registries in France, incidence rates between 2009 and 2011 were 0.59 per 100,000 men (95% CI: 0.50–0.68) and these rates have remained stable since 1989 [16].
In the USA, the incidence of penile cancer is affected by race and ethnicity, with the highest incidence in white Hispanics (1.01), followed by Alaskans and Native American Indians (0.77), African Americans (0.62) and white non-Hispanics (0.51), per 100,000 males, respectively. The overall age-adjusted incidence rate decreased between 1973 and 2002; per decade from 0.84 (1973–1982), to 0.69 (1983–1992), and 0.58 (1993–2002) per 100,000 males, respectively [17]. An increasing trend, slightly surpassing the previous incidence rates, was described using the Surveillance, Epidemiology and End Result (SEER) 2000–2016 data [18], showing an estimated annual percent change of +3.5% from 2004-2016 [19].
The incidence increases with age [15,20], with a peak in the sixth decade but it does occur in younger men [21]. Penile cancer is common in regions with a high prevalence of human papillomavirus (HPV), and approximately one-third to half of cancer cases are attributed to HPV-associated carcinogenesis [22,23]. There are no reports linking this cancer to human immunodeficiency virus (HIV) or acquired immune deficiency syndrome (AIDS).
In summary, it seems that a slight increase in incidence is seen in Western/developed countries, most likely caused by higher infection rates of HPV which is a trend also observed in oropharynx carcinoma [24].
Figure 3.1: Annual incidence rate (world standardised) by world area [25]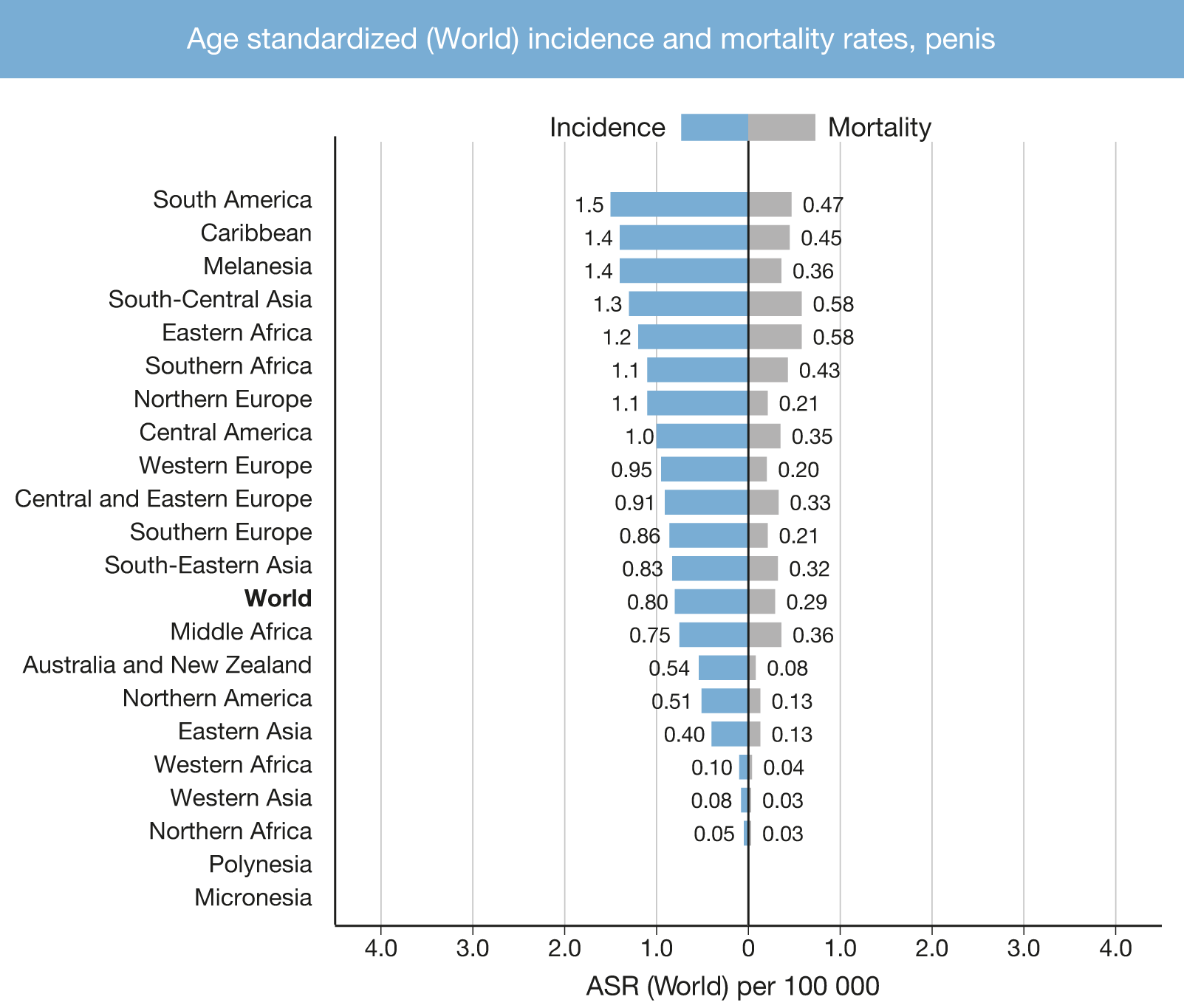
3.3. Risk factors, prognosis, and prevention
Several risk factors for penile cancer have been identified, such as phimosis, chronic penile inflammation, lichen sclerosus, smoking, ultraviolet A phototherapy, and low socio-economic status, amongst others [26].
Patient outcome is influenced by clinical and histologic features. United States SEER data from 18 cancer registries indicated an overall 5-year relative survival of 67% with no significant changes when comparing 5-year spans between 2000–2014. Patients with localised disease showed the best outcome with up to 81% 5-year relative survival. Patients with distant metastases have the worst outcomes with only 16% 5-year relative survival [27].
Human papilloma virus infection is the main risk factor for penile cancer [28]. Human papilloma virus deoxyribonucleic acid (DNA) has been identified in intraepithelial neoplasia and invasive penile cancer tissue samples. The HPV virus interacts with oncogenes and tumour suppressor genes (p16, P53, Rb genes) [29,30]. The rate of HPV-positivity differs between different histological subtypes of penile SCC. Human papilloma virus is a co-factor in the carcinogenesis of some subtypes of penile SCC, while others are not related to HPV. The risk of penile cancer is increased in patients with condyloma acuminata [31]. A SR of 52 studies concluded that the overall HPV prevalence in penile cancer is 50.8% (95% CI: 44.8–56.7). Among HPV-associated carcinomas, basaloid carcinoma showed the highest prevalence (84%) followed by warty-basaloid carcinoma (75.7%) and warty carcinomas (58.7%). In histologically HPV-independent carcinomas, HPV prevalence was 19.4%. The most frequent HPV genotypes were HPV16 (68.3%, 95% CI: 58.9–77.1), followed by the low-risk HPV6 genotype (8.1%, 95% CI: 4.0–13.7) [23].
In early studies, HPV has shown an inconsistent association with prognosis. In one study, a significantly better 5-year disease-specific survival (DSS) was reported for HPV-positive vs. HPV-negative cases (93% vs. 78%) [32], while no difference in lymph node (LN) metastases and 10-year survival was reported in another study [33]. This variable relationship with outcome remains unexplained but some studies suggested that it can be related to specific treatment [34] and linked to different histologic subtypes [35]. A meta-analysis published in 2018 [36] reported a pooled HR of 0.61 for penile cancer HPV-positive cases, which is in line with head, neck [37] and anal cancers [38], with a HR of 0.34 and 0.54, respectively. Positivity for p16 immunohistochemistry (IHC), a surrogate for HPV activity, showed a prognostic value for DSS (hazard ratio [HR]: 0.45) based on two meta-analyses [36,39]. Similar trends were reported in vulvar and anal cancers [38,40].
There is no significant association between the incidence of penile and cervical cancer, although half of penile cancer and virtually all cervical cancer cases are linked to HPV [41]. Female sexual partners of patients with penile cancer have not been found to have an increased incidence of cervical cancer [42].
At present, except in a limited number of countries, there is no general recommendation for HPV vaccination in males because of the different HPV-associated risk patterns in penile- and cervical cancer. A meta-analysis showed that the incidence of anal (risk ratio [RR]: 0.42), oral (RR: 0.16), and cervical HPV infections (RR: 0.22) were reduced in vaccinated groups when compared against control groups, indicating that HPV vaccination leads to the prevention of HPV infection [43]. Human papilloma virus vaccination in males showed more than 50% efficacy against anal intraepithelial lesions but no meaningful estimates were obtained for penile, anal, and head and neck invasive carcinomas [44]. Since up to 50% of invasive penile carcinomas and 80% of pre-neoplastic lesions are HPV-associated, HPV vaccination is encouraged [45].
Phimosis is strongly associated with invasive penile cancer [46-49], due to associated chronic infections. However, smegma is not a carcinogen [48]. The incidence of lichen sclerosus is relatively high in penile cancer patients but is not associated with adverse histopathological features, including penile intraepithelial neoplasia (PeIN). Other epidemiological risk factors are cigarette smoking, low socio-economic status, and a low level of education [47,49].
Neonatal circumcision reduces the incidence of penile cancer; however, it does not seem to reduce the risk of PeIN [46]. The lowest incidence of penile cancer is reported in Israeli Jews (0.3/100,000/year). One matched-pair case-control study reported that the protective effect of neonatal circumcision against invasive penile cancer (OR: 0.41) was much weaker when the analysis was restricted to men without a history of phimosis (OR: 0.79, 95% CI: 0.29–2) [46].
3.4. Pathology
Squamous cell carcinoma accounts for over 95% of penile malignancies. It is not known how often SCC is preceded by premalignant lesions [50-53]. Penile intraepithelial neoplasia is considered the precursor lesion of penile SCC, PeINs are classified into HPV-independent, known as differentiated PeIN, HPV-associated, following the same scheme as the invasive counterparts (see Table 3.2). Clinical terms such as ‘Erythroplasia of Queyrat, Bowenoid papulosis and Bowen’s disease’ are discouraged, based on the 2022 WHO classification [8,54].
Different histological types of penile SCC with different growth patterns, clinical aggressiveness and HPV associations have been identified (see Table 3.1). Numerous mixed forms exist such as the warty-basaloid form, with 50–60% the most common mixed form, the usual-verrucous (hybrid), usual-warty, usual-basaloid and the usual-papillary, as well as other rarer combinations.
Other malignant lesions of the penis, all much less common than penile SCC, are melanocytic lesions, mesenchymal tumours, lymphomas, and metastases. Penile metastases are frequently of prostatic, urinary bladder or colorectal origin [55]. Different types of penile sarcoma have been reported [8].
Table 3.2: Classification of penile intra-epithelial neoplasia
HPV-independent |
oDifferentiated PeIN |
HPV-associated PeIN |
oCommon patterns: basaloid (undifferentiated), warty (condylomatous), and mixed |
oOther (less frequent) patterns: pagetoid, clear cell, and spindle cell histology |
3.4.1. Gross handling of pathology specimens
Tissue sections determine the accuracy of histological diagnosis. Small lesions should be fully included, bigger lesions should have at least 3-4 blocks of tumour with the anatomical landmarks. Specimens should be properly oriented by the surgeons and, in case of circumcision or glans resurfacing, properly pinned to allow clear evaluation of the resection margins. Penectomy specimens must be canalised through the urethra and cut longitudinally in two halves for the evaluation of invasion of the penile structures. In larger tumours identification of distal urethra on gross (as also microscopy) may be difficult. Whole-mount inclusion and sections are recommended as they provide a better background for the appropriate identification of anatomical structures that can improve accurate staging, with a minimal increase in cost [56]. Sentinel LN should be evaluated according to a standardised IHC protocol [57] for detection of micro-metastases; lymphadenectomy/lymph node dissection (LND) specimens should be inked, and the LNs evaluated properly since extra-capsular extension profoundly influences nodal staging and treatment decisions. Second-opinion pathology review is highly desirable for this rare tumour entity [58], as is setting up comprehensive referral centres for penile cancer management on a national level [58,59].
3.4.2. Pathology report
For standardisation and data collection purposes the dataset template from the International Collaboration on Cancer Reporting (ICCR) should be used when possible. The pathology report must include the anatomical site of the primary tumour, the histological type of SCC, grade, perineural invasion, depth of invasion, vascular invasion (venous/lymphatic), pattern of invasion, urethral invasion, invasion of corpus spongiosum/corpora cavernosum, surgical margins and p16 IHC results [60-63] (Table 3.3). The confirmation of the presence of HPV in the specimen (e.g., polymerase chain reaction [PCR], in-situ hybridization [ISH] for viral DNA/ribonucleic acid [RNA]) is desirable but currently only carried out in research settings.
Table 3.3: Information to include in pathology reports for penile carcinomas
Type of information* | Recommended | required |
Clinical information Prior treatments (topic, radiotherapy, chemotherapy) | x | |
Surgical procedure | x | |
Tumour localisation Anatomic structures involved externally (e.g.: foreskin, glans, etc.) and in depth (e.g.: dartos, corpus spongiosum, etc.) | x | |
Macroscopic tumour dimension Size of tumour Maximum thickness | x | |
Photographic documentation | x | |
Block identification with description of the localization of the samples | x | |
Histological tumour type | x | |
Histological grade | x | |
Microscopic maximum dimensions Depth of invasion (i.e., millimetres from basement membrane to deepest point of invasion) Combination of gross and microscopic if large tumours | x | |
Extent of invasion (microscopic confirmation of all the involved anatomic structures) | x | |
Tumour invasion front (Broadly-based pushing, destructive but well-delineated, destructive irregular/finger-like invasion/tumour budding) [64-66] | x | |
x | ||
Perineural invasion | x | |
Margin status in mm (margins as per specimen) | x | |
Lymph node status Size of largest nodal tumour deposit (not LN size) Total number of LNs, number of positive LNs, extra-capsular spread (ECS), inguinal or pelvic, to be reported in every site separately | x | |
pTNM Stage | x | |
HPV assessment (at least p16 IHC based) | x |
* See also ICCR dataset: https://www.iccr-cancer.org/datasets/published-datasets/urinary-male-genital/penis/.
3.4.3. Grading
The tumour, node, metastasis (TNM) classification for penile cancer includes tumour grade based on its prognostic relevance. Tumour grading in penile cancer has been shown to be highly observer-dependent and can be problematic, especially in large tumours which may be heterogeneous. This may have implications on the clinical management, as there may be discordance between biopsy and resection grading [50]. Inter-observer agreement varies according to the experience and specialisation of the pathologist. In general, inter-observer agreement is poor to moderate (Fleiss’ kappa 0.07–0.55) [69]. Nevertheless, until a new methodology to grade penile SCC is developed, grading based on the WHO/The International Society of Urological Pathology (ISUP) classification is recommended (see Table 3.4) with grade 3 and sarcomatoid being considered as poorly differentiated.
Table 3.4: Grading recommendations for penile SCC
Feature | Grade 1 | Grade 2 | Grade 3 | Sarcomatoid |
Cytological atypia | Mild | Moderate | Anaplasia | Sarcomatoid |
Keratinisation | Usually abundant | Less prominent | May be present | Absent |
Intercellular bridges | Prominent | Occasional | Few | Absent |
Mitotic activity | Rare | Increased | Abundant | Abundant |
Tumour margin | Pushing/well | Infiltrative/ill defined | Infiltrative/ill defined | Infiltrative/ill defined |
3.4.4. Pathological prognostic factors
Pathological subtype, peri-neural invasion, lymphovascular invasion [67], depth of invasion and grade in the primary tumour are strong predictors of poor prognosis and high cancer-specific mortality [70]. Higher grade and lymphovascular invasion are predictors of metastatic spread. Lymphovascular space involvement/invasion is often seen in advanced stages but may also be seen in early invasive tumours of high grade and some histologic subtypes [71,72]. The extent of LN metastasis and extracapsular spread are also strong predictors of prognosis.
Urethral invasion is not considered a prognostic factor (UICC, 8th Edn) [73]. Nevertheless, invasion of the more proximal urethra can signify a highly aggressive SCC with a poor prognosis probably due the invasion of subjacent erectile corpora (see Table 4.1). A SR found that invasion of the corpus spongiosum (pT2) showed better cancer-specific survival (CSS), but no overall survival (OS) benefit compared to invasion of the corpora cavernosa [74]. A modified pT2/T3 has been proposed, taking into consideration high-grade, lymphovascular- and perineural invasion features in response to this inconsistency [75]. Extra-capsular extension in even one single LN carries a poor prognosis and is denoted as pN3 [76-78].
Chaux et al., suggested a prognostic index which incorporates grade, anatomical level of infiltration and perineural invasion to predict the likelihood of inguinal LN metastases and 5-year survival [79]. Sali et al., proposed a histopathological risk scoring incorporating grade, anatomical level of involvement and replaced perineural invasion with pattern of infiltration [66]. Other clinical-, pathological- and radiological scores, as well as nomograms have been described but none of these have been comparatively validated which precludes making a recommendation.
3.4.5. Penile cancer and HPV
In the 2022 WHO classification the presence of HPV is a key determinant for the broad classification of penile SCC [8]. However, in most clinical settings, standard molecular assessment of HPV status is not available.
p16 IHC is used as a surrogate for high-risk HPV genotype presence and marker of oncogenic activity. In the absence of more advanced techniques, it is helpful in assigning penile SCC to HPV-associated subtypes. The p16 IHC overall positivity in penile cancer was 41.6% [23]. Higher positivity was seen in morphological HPV-associated SCCs (85.8%) as compared with HPV-independent SCCs (17.1%)[23]. Comparing with RNA ISH, p16 IHC showed a sensitivity of 100% and a specificity of 71%, the latter improved to 89% when considering a high intensity for p16 IHC positivity [80]. These data indicate that sensitivity, specificity, and predictive values for HPV positivity can be improved using the stringent p16 IHC cut-off suggested by Cubilla et al. [81] (Figure 3.2). The ISUP reported that 80% of their respondents during a consultation conference on molecular pathology of urogenital cancers used p16 IHC to separate HPV-associated from HPV-independent PeIN and SCCs and made recommendations on the use of p16 IHC [82].
Fig 3.2: Patterns of p16 expression. (A) no staining; (B) mosaic staining pattern: (C) en-bloc staining pattern. Only (C) is considered positive for p16.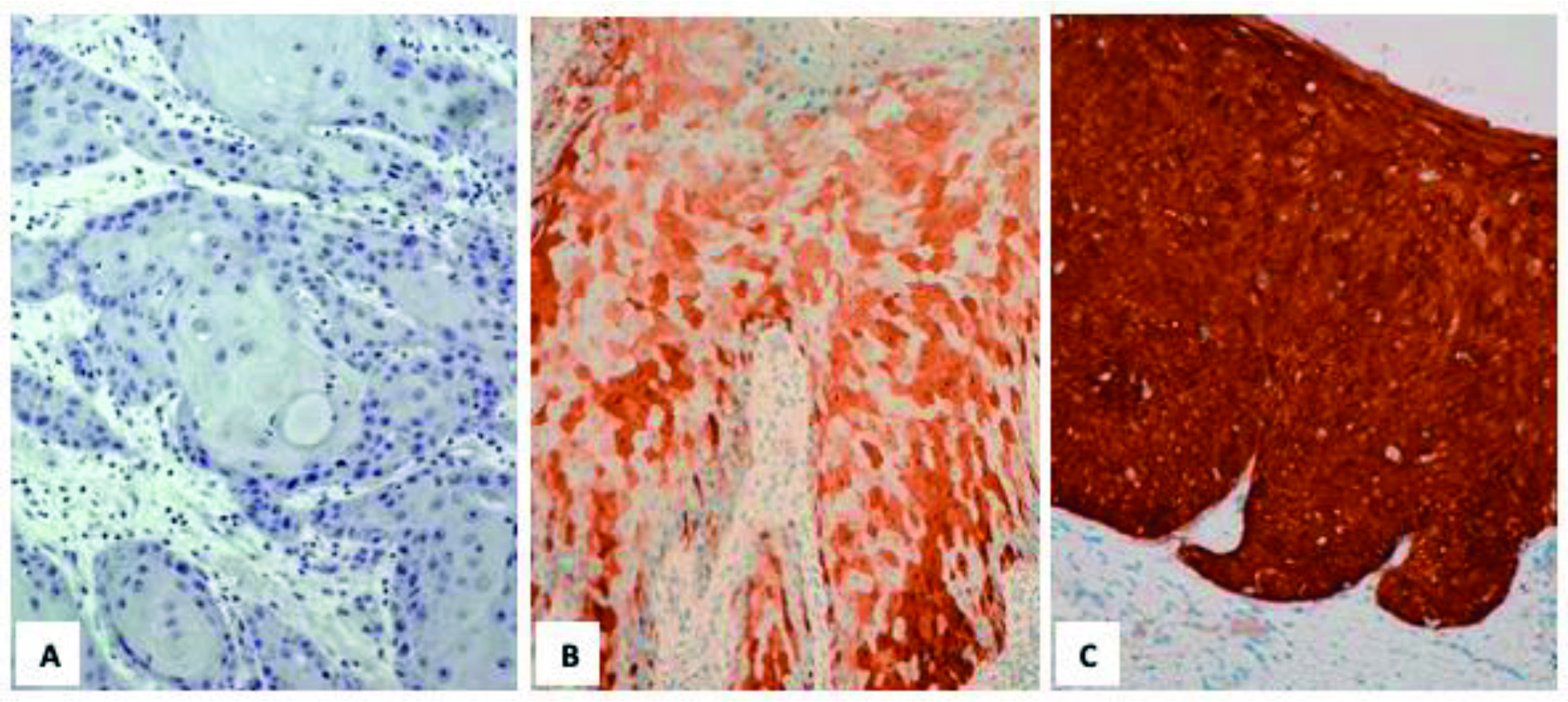
3.4.6. Penile biopsy: pathological and technical considerations
The quality of biopsy is important [50]. In most cases, acquiring a punch biopsy (e.g., 2–3 mm) under local anaesthesia is sufficient to confirm the diagnosis. In biopsies with an average size of 1 mm, it was difficult to evaluate the depth of invasion in 91% of cases [50]. Furthermore, vascular and lymphatic tumour emboli were detected in only 9–11% of cases [50]. Therefore, in cases where assessment of depth of invasion is necessary, an incisional biopsy which is deep enough to properly assess the degree of invasion and stage is preferable.
3.4.7. Summary of evidence and guidelines for the pathological assessment of tumour specimens
Summary of evidence | LE |
Incidence of penile cancer varies according to geographical location, race and ethnicity. | 2a |
Western developed countries have seen a slight increase in incidence, which may be caused by higher HPV infection rates. | 2a |
In analogy to other HPV-associated cancers, HPV status may influence DSS of penile cancer, but more data is needed, underlining the importance of routine assessment of HPV status in all penile cancer patients. | 2b |
Recommendations | Strength rating |
The pathological evaluation of penile carcinoma specimens must include the pTNM (see Chapter 4) stage and an assessment of tumour grade. | Strong |
The pathological evaluation of penile carcinoma specimens must include an assessment of p16 by immunohistochemistry. | Strong |
The pathological evaluation of penile carcinoma specimens should follow the ICCR dataset synoptic report. | Strong |
ICCR = International Collaboration on Cancer Reporting.